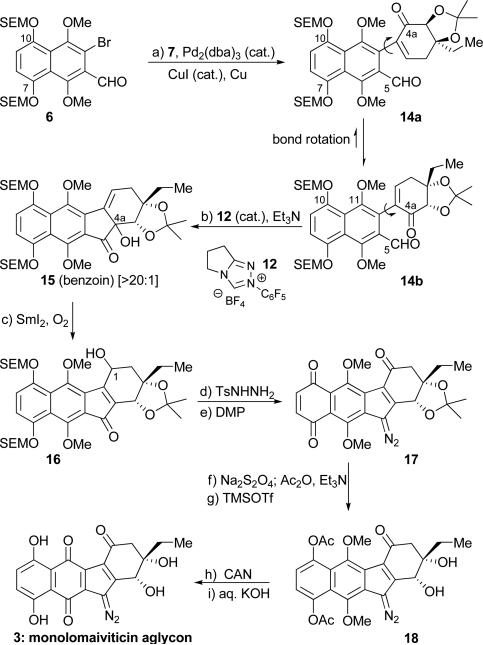
Scheme 3.
Completion of the synthesis of monolomaiviticin aglycon (3). Reagents and conditions: a) 6 (1.0 equiv), 7 (1.5 equiv), CuI (0.4 equiv), Pd2(dba)3 (0.1 equiv), Cu (10.0 equiv), DMSO, 65 °C, 3 h, 69 %; b) 12 (0.2 equiv), Et3N (2.0 equiv), CH2Cl2, 42 °C, 18 h, 70 %, 3:1 mixture of diastereomers, >20:1 benzoin:Stetter; c) SmI2 (4.0 equiv), MeOH (10.0 equiv), THF, –78 °C, 5 min; –78→25 °C; O2 (balloon), 25 °C, 18 h, 76 %, 1.5:1 mixture of diastereomers; d) TsNHNH2 (5.0 equiv), aq. HCl (1 M) : iPrOH (1:100), 25 °C, 18 h, 91 %, 1:1 mixture of E:Z isomers of a 1.5:1 mixture of diastereomers; e) DMP (5.0 equiv), CH2Cl2, 25 °C, 1.5 h, 62 %; f) Na2S2O4 (5.0 equiv), EtOAc, H2O, 25 °C, 5 min; Ac2O (10.0 equiv), Et3N (10.0 equiv), DMAP (1.0 equiv), CH2Cl2, 25 °C, 20 min, 91 %; g) TMSOTf (5.0 equiv), CH2Cl2, 25 °C, 20 min, 96 %; h) CAN (3.0 equiv), MeCN, pH 7 phosphate buffer, 25 °C, 20 min, 96 %; i) aq. KOH (1 M), THF, H2O, 25 °C, 30 min, 95 %. Ts = 4-toluenesulfonyl, DMP = Dess–Martin periodinane, Ac2O = acetic anhydride, DMAP = 4-dimethylaminopyridine, CAN = cerium ammonium nitrate.