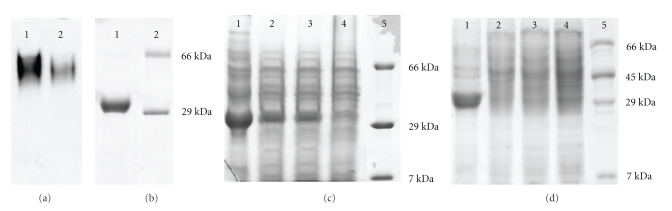
Figure 1.
Polyacrylamide gel electrophoresis of SBL samples. (a) Product from affinity chromatography purification procedure in silver-stained nondenaturing PAGE at pH 8 and with a polyacrylamide concentration of 7.5%. Lane 1: commercially obtained SBL (Sigma Chemical Co.), lane 2: product from affinity purification. (b) The same SBL fraction in silver-stained SDS-PAGE. Lane 1: final SBL purification fraction; lane 2: molecular weight markers. (c) The same SBL fraction in Coomassie blue-stained SDS-PAGE after incubation with B. japonicum USDA 110 cells and centrifugation. Cells were incubated with 10 μg ml−1 SBL for 12 hours and centrifuged at 10.000 × g for 20 minutes without further processing (lane 1), or after three cycles of resuspension in different solutions, agitation at 4°C 10 minutes and centrifugation at 10.000 × g for 20 minutes. Lane 2: resuspension in low salts buffer, containing 3.0 mM KCl, 1.5 mM KH2PO4, 68.0 mM NaCl, and 9.0 mM NaH2PO4; lane 3: resuspension in 1 M NaCl; lane 4: resuspension in PBS containing 1 M galactose; lane 5: molecular weight markers. (d) Comparison of the protein profiles from USDA 110 and the mutant ΔP22 after incubation with or without 10 μg ml−1 SBL for 12 hours followed by centrifugation at 10.000 × g for 20 minutes. Lane 1: USDA 110 with SBL. Lane 2: USDA 110 without SBL. Lane 3: ΔP22 with SBL. Lane 4: ΔP22 without SBL. Lane 5: Molecular weigh markers.