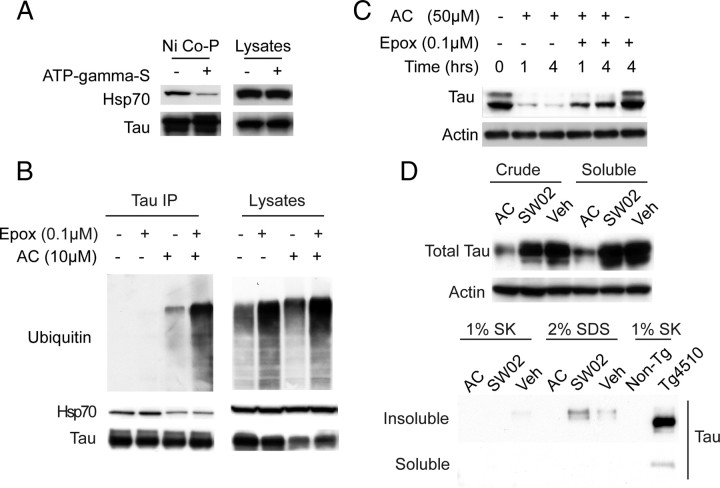
Figure 3.
Nucleotide state of Hsp70 regulates tau binding and inhibitor-mediated reductions in tau occur via proteasomal degradation, not by changes in tau aggregation. A, Lysates from cells overexpressing Hsp70 were supplemented with His-tagged tau and incubated with or without ATP-γ-S for 30 min. Coprecipitation with Ni-agarose beads (Ni Co-P) shows reduced binding of tau to Hsp70 in the presence of the nonhydrolyzable ATP analog. B, Ubiquitination of tau is evident in immunoprecipitates from HEK tau transfectants following treatment with AC (10 μm). Epoxomicin (Epox, 100 nm) treatment for 6 h further increased the amount of ubiquitinated tau. Hsp70 binding to tau was also reduced by AC treatment, regardless of epoxomicin treatment. C, Tau levels 1 and 4 h after epoxomicin (100 nm) and AC (50 μm) treatment of HeLa tau transfectants. Actin was used to control for loading in all panels. D, Equivalent tau levels are observed in both crude and Tris-saline soluble preparations of HeLa tau transfectant lysates treated with AC (50 μm) or SW02 (50 μm) for 1 h. Absence of tau from soluble or insoluble fractions of Sarkosyl (SK) or SDS extracts prepared from insoluble pellets. Tg4510 brain Sarkosyl extract is shown as a procedural control.