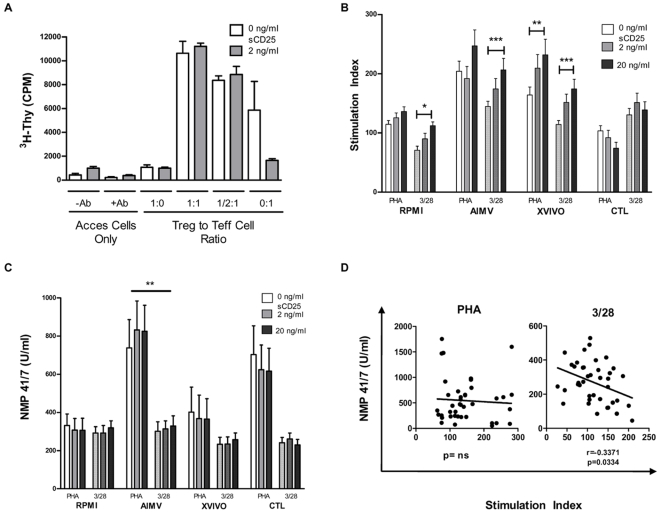
Figure 6. Addition of soluble CD25 differentially effects proliferation of Teff cells and PBMC.
A suppression assay was set up under serum-free media ((A), open bars), or in the presence of a human recombinant sCD25 protein ((A), closed bars). Addition of sCD25 did not significantly restore Teff cell responses or the anergic properties of Treg in serum-free conditions (p = NS for all Treg to Teff cell ratios, 1∶0, 1∶1, ½∶1, and 0∶1). In the 0∶1 condition, sCD25 did appear to reduce proliferation. sCD25 was added to PBMC cultures (n = 10) in the presence of increasing concentrations of sCD25, with anti-CD3/28 microbeads ((B), open bars) or PHA ((B), hatched bars) stimulation. Proliferation increased in some culture conditions and decreased in others. Statistically significant increases were seen by ANOVA under 3/28 stimulation in RPMI (p = 0.05), AIMV (p = 0.001), and XVIVO (p = 0.001) media, and under PHA stimulation in XVIVO medium (p = 0.01) when comparing 0 and 20 ng of sCD25 added. Comparisons among the same stimulation showed that cells cultured in RPMI and AIMV media had significant differences in proliferation regardless of the amount of sCD25 added, with both PHA and CD3/28 stimulation. Under PHA stimulation, CTL cultures proliferated less than either XVIVO or AIMV cultures. To determine if this differential proliferation was due to altered cell death, we measured the amount of nuclear matrix proteins (NMPs) released into the supernatant by dying cells (C). Addition of sCD25 did not result in a dose-responsive increase in cell death. The outcome varied depending on the culture media and stimulus. Plotting the stimulation index versus the production of NMP showed a negative correlation between proliferation and cell death under CD3/28 stimulation ((D), bottom panel). *p<0.05, **p<0.01, and ***p<0.001.