Abstract
Objectives
To identify the variables that predict death/physiologic BPD in preterm infants with severe respiratory failure.
Study Design
The study was a secondary analysis of data from the NICHD Neonatal Research Network trial of inhaled nitric oxide (iNO) in preterm infants. Stepwise logistic regression models and Classification and Regression Tree (CART) models were developed for the outcome of death or physiologic BPD (O2 at 36 weeks’ postmenstrual age).
Results
Death and/or BPD was associated with lower birth weight, higher oxygen requirement, male gender, additional surfactant doses, higher oxygenation index, and outborn status, but not the magnitude of response in PaO2 to iNO. The positive predictive value of the CART model was 82% at 95% sensitivity.
Conclusions
The major factors associated with death/BPD were an increased severity of respiratory failure, lower birth weight, male gender, and outborn status, but not the magnitude of initial response to iNO.
Keywords: Logistic models, Predictive value of tests, ROC curve
INTRODUCTION
Preterm infants with respiratory failure are at high risk of mortality or morbidity. Inhaled nitric oxide (iNO) may improve ventilation-perfusion mismatch and oxygenation, lower pulmonary arterial pressures, reduce lung inflammation, and thereby attenuate the pathophysiology of respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD). The recent NICHD trial of iNO in preterm infants did not show a difference in the primary outcome of bronchopulmonary dysplasia (BPD) or death between the control and the iNO groups.1 However, post hoc analysis of data from this trial indicated that iNO may benefit very low birth weight (VLBW) infants with certain characteristics (birth weight (BW) >1000 g) but may worsen outcome in other VLBW infants (e.g. BW <1000 g, on conventional ventilation).1 Other recent trials of iNO in premature infants2,3,4 indicate that iNO may benefit some premature infants, especially when used for longer durations.4 It is of clinical importance to determine and to identify clinical variables that are associated with a worse outcome in order to design new clinical observational and interventional studies. Previous small studies have indicated that a lack of initial response was predictive of death.5 In the NICHD trial, response to study gas was defined by the change in PaO2 between baseline and 30 minutes of initiating iNO without any alterations in ventilator or oxygen settings.1 A complete response was an increase of more than 20 mm Hg; a partial response, an increase of 10 to 20 mm Hg; and no response, an increase of less than 10 mm Hg. However, the degree of response was arbitrarily defined, and it is possible that a different magnitude of response (e.g. 30 or 40 mm Hg) may be associated with improved survival and a decreased incidence of BPD.
Classification and Regression Tree (CART) analysis is a statistical method that develops intuitive diagrams for identification of risk factors, prognosis, or similar patterns in data. By recursive partitioning and automatic selection of optimal cut-points of variables, a classification tree is developed with a series of binary splits. When applied to data containing patients with the outcome of interest (cases) and those without (controls), each binary split in a classification tree yields two subgroups, one with a higher proportion of cases and the other with a higher proportion of controls. A major advantage of CART analysis as compared to other statistical methods such as regression analysis is that no empirical cut-points for any of the variables are chosen, but the most optimal cut-point for each variable is determined by the software using the available data. Also, the more closely associated a variable is in relation to outcome, the higher it is on the decision tree, and this facilitates the identification of the relative importance of variables. CART models are designed to handle a large number of predictor variables without making assumptions about the relative importance of each variable, and this technique does not assume that data are linearly related. CART analysis is an excellent method for initial data exploration and the results are resistant to the influence of outlier data. Decision trees may also be easier for clinicians to use, as compared to regression models, as they do not require equations or calculations but merely following the tree from beginning to end, with decisions being made at each node based on available clinical data.
Using the two complementary approaches of stepwise logistic regression and CART analysis, our objective was to determine if the magnitude of initial improvement in PaO2 in response to iNO predicts death and/or BPD and to identify other variables that predict death and/or BPD. We hypothesized that a smaller improvement in oxygenation and an increasing severity of respiratory failure (as measured by OI) would be associated with death and/or BPD.
METHODS
This study was a secondary analysis of data from the NICHD Inhaled Nitric Oxide for Premature Infants with Severe Respiratory Failure trial.1 In this trial, 420 neonates born at less than 34 weeks of gestation, with birth weights of 401 to 1500 g, and with severe respiratory failure more than four hours after treatment with surfactant were randomly assigned to receive placebo (simulated flow) or iNO (5 to 10 ppm) in one of the participating centers (Appendix). 1 Infants with a response (an increase in the PaO2 of more than 10 mm Hg) were managed according to protocol. Treatment with study gas was discontinued in infants who did not have a response (an increase in the PaO2 of less than 10 mm Hg).1 The primary outcome for this ancillary study was death (defined as death before discharge to home or within 365 days among hospitalized infants) or physiologic bronchopulmonary dysplasia (defined as requiring supplemental oxygen at 36 weeks gestational age; infants not on mechanical ventilation and receiving less than 30 percent oxygen were assessed by performing a stepwise reduction in oxygen delivery to the lowest oxygen concentration at which the oxygen saturation measured by pulse oximetry remained at least 90 percent).1,6 Unlike the main trial which defined BPD as oxygen use at 36 weeks, this ancillary study used the physiologic BPD endpoint in an attempt to reduce inter-center variation in the assessment and diagnosis of BPD.6
In order to determine if the magnitude of initial improvement in PaO2 in response to iNO predicts death/BPD and to identify the variables that predict death/BPD, we performed stepwise logistic regression using SAS software (SAS Institute Inc, Cary, NC)) on the full set of 420 study infants and on the iNO and control groups separately, with death or physiologic BPD as the outcome. The iNO and control groups were evaluated separately in addition to evaluating the full data set, in order to determine if there were differences in predictors when the infants had been exposed to iNO. The variables listed for inclusion in the models are shown in Table 1. These variables were the variables available at study gas initiation, as well as the magnitude of response to study gas (as a continuous variable). The treatment/control variable was included as an independent variable during analysis of the full data set. Variables were allowed to enter/stay in the model at p<0.2. Taking into consideration the sample size of the iNO group, and the magnitude of the variation of the response in PaO2 to iNO, we estimate that we would have been able to detect a 50% difference in response between the infants who had a good outcome (no BPD/death) and those with a bad outcome (BPD/death) at 80% power, with a p-value of <0.05.
TABLE 1.
Variables used for development of stepwise logistic regression models to predict death/BPD
| Category | Variable included for model development |
Variable description |
|---|---|---|
| Demographics | Birth weight | Birth weight (100g increments) |
| Gestation | Gestational age in completed weeks | |
| Gender | Gender | |
| Race | Race | |
| Outborn | Inborn vs. Outborn | |
| C-section | Mode of delivery | |
| Randomization age | Age at Randomization in hours | |
| Diagnosis | Primary diagnosis at randomization | |
| Illness severity | Mean FiO2 | Mean FiO2 at inclusion |
| Mean OI | Mean of inclusion OIs | |
| Response to iNO* | Treatment/Control | Allocation to treatment or control groups |
| PaO2 Diff 5 | Change in PaO2 Baseline to 5 ppm iNO | |
| PaO2 Max Diff | The greater of the change in PaO2 Baseline to 5 ppm and the change in PaO2 5 ppm to 10 ppm |
|
| Disease conditions prior to study initiation |
Air leak | Air leak (e.g. pneumothorax, interstitial emphysema) |
| GI Bleed | Gastrointestinal bleeding | |
| Oozing | Prolonged oozing of blood from iv sites | |
| Other bleeding | Other sites of bleeding | |
| Pulmonary hemorrhage | Clinically diagnosed pulmonary hemorrhage | |
| Seizures | Seizures | |
| Therapies prior to study initiation |
Ventilation mode | Mode of ventilation (conventional vs. high frequency) |
| Paralysis | Use of muscle relaxants | |
| Steroids | Use of postnatal steroids | |
| Surfactant dose | Surfactant doses administered | |
| Vasopressors | Vasopressor (e.g. dopamine, dobutamine) use | |
| Volume | Volume administration | |
| Sedation | Use of sedation/analgesia |
The treatment/control group allocation variable was used in the full data set analysis, and the response to iNO was used in analysis of the treatment group which received iNO.
Prognostic algorithms were developed using Classification and Regression Tree (CART) analysis. CART models were created using AnswerTree software (SPSS, Chicago, IL) that performed recursive partitioning and automatic selection of optimal cut-points of variables. The same variables used for the stepwise logistic regression model (Table 1) including the treatment/control variable and the magnitude of response to iNO were used for the CART model development. The maximum tree depth was empirically set at 5 levels, with a minimum number of 40 observations in each parent (upper) node and 20 observations in each child (lower) node, in order to reduce errors due to small sample sizes.
RESULTS
The iNO and placebo groups were comparable, with the mean gestational age of 26 weeks (SD ±2 weeks), and the birth weight approximately 840g.1 The rate of death or bronchopulmonary dysplasia (physiologic BPD) was 76% in the iNO group, as compared with 78% in the placebo group (p NS). The rates of death (52% in iNO group vs. 44% in placebo group, p NS) and of physiologic BPD in survivors (54% in iNO group vs. 63% in placebo group, p NS) were also not statistically different.
Regression models
Randomization to receive iNO and the magnitude of response in PaO2 to iNO were not identified as being associated with the outcome of death and/or BPD when all enrolled infants were analyzed (Table 2). Death and/or BPD was associated with lower birth weight, higher oxygen concentration (FiO2) at enrollment, male gender, additional surfactant doses, higher oxygenation index (OI), and outborn status (Table 2). The variables selected by stepwise regression for the iNO group and control group were roughly comparable, but higher birth weight was significantly associated with lower death/BPD only in the iNO group (OR 0.65 per 100g, (0.56 – 0.76)) (Table 2).
Table 2.
Variables identified by stepwise logistic regression models as associated with death/BPD. Variables were allowed to enter/stay in the model at p<0.2
| Full data set | ||
|---|---|---|
| Stepwise logistic regression |
Variables added | Odds ratio ( 95% CI ) |
| First Step | Birth weight (per 100g) | 0.78 (0.71 – 0.85) |
| Second Step | Mean FiO2 | 3.14 (0.72 – 13.63) |
| Third Step | Male gender | 2.54 (150 – 4.28) |
| Fourth Step | Surfactant dose | 1.44 (1.06 – 1.96) |
| Fifth Step | Mean OI (per unit) | 1.03 (1.00 – 1.05) |
| Sixth Step | Outborn | 1.73 (0.91 – 3.30) |
| c statistic for model = 0.75 | ||
| Treatment (iNO) group | ||
|---|---|---|
| Stepwise logistic regression |
Variables added | Odds ratio (95% CI) |
| First Step | Birth weight (per 100g) | 0.65 (0.56 – 0.76) |
| Second Step | Mean FiO2 | 26.54 (3.88 – 181.48) |
| Third Step | Age at randomization (hours) | 0.97 (0.96 – 0.99) |
| Fourth Step | Surfactant dose | 2.21 (1.27 – 3.84) |
| Fifth Step | Seizures | 0.28 (0.04 – 1.85) |
| c statistic for model = 0.84 | ||
| Control group | ||
|---|---|---|
| Stepwise logistic regression |
Variables added | Odds ratio (95% CI) |
| First Step | Male gender | 4.67 (2.13 – 10.27) |
| Second Step | Gestational age (per week) | 0.86 (0.73 – 1.02) |
| Third Step | High frequency ventilation | 0.39 (0.18 – 0.86) |
| Fourth Step | Outborn | 2.34 (0.91 – 6.04) |
| Fifth Step | Air Leak | 0.33 (0.11 – 0.96) |
| Sixth Step | Mean OI (per unit) | 1.02 (0.99 – 1.05) |
| Seventh Step | Change in PaO2 (baseline to 5 ppm study gas) |
0.98 (0.96 – 1.00) |
| Eighth Step | PaO2 Max Diff | 1.02 (1.00 – 1.04) |
| c statistic for model = 0.76 | ||
CART models
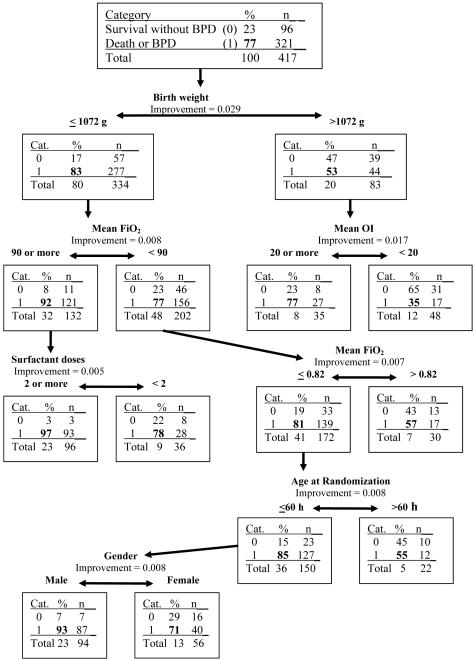
Predictive variables which are more strongly associated with the outcome are shown higher on the decision tree (Figure 1). Infants with birth weight >1072 g were less likely to develop death and/or BPD (53% death/BPD vs. 83% for ≤1072 g). In infants >1072 g, an OI of 20 or more predicted worse outcome (77% vs 35% for OI of <20). For infants ≤1072 g, FiO2 of 90% or more predicted worse outcome (92% vs. 77% with FiO2 <90). Infants with FiO2 of 90% or more receiving 2 or more doses of surfactant had a worse outcome (97% vs. 78%). The positive predictive value of this CART model was 82% at 95% sensitivity, with a negative predictive value of 65% and specificity of 32%. Randomization to the iNO group and the magnitude of response in PaO2 to iNO were not identified as being associated with the outcome of death and/or BPD.
Figure 1. CART model for death/physiologic BPD.
The dichotomous outcome of death or physiologic BPD at 36 weeks’ post-menstrual age is predicted by this decision tree. In each node (rectangle), the category “0” or “1” refers to the absence or presence of death/BPD, respectively, and the percentages and “n” refer to the infants in each of the categories. The increment in positive predictive value with the variable under consideration is shown as the “improvement” (e.g. 0.09 = a 9% increase in positive predictive value).
DISCUSSION
The regression and CART models identified factors associated with death and/or BPD in premature infants with respiratory failure. The magnitude of improvement in PaO2 in response to iNO was not found to be associated with death and/or physiologic BPD, indicating that the initial response to iNO in premature infants with severe respiratory failure may not be a good gauge of whether iNO should be continued. The main trial1 did not demonstrate any benefit of iNO on BPD/death in these infants despite initial improvement in oxygenation, and our study extends these observations by showing that even if there were a greater initial response to iNO, there was no reduction in death/BPD. In addition, we confirmed the previous finding that higher birth weight was a predictor in the iNO but not control infants. In general, both the regression and CART models identified smaller infants with a greater severity of illness as more likely to either die or develop BPD. While the findings that smaller and sicker infants have a worse outcome is not surprising, the CART analysis ranks the importance of these predictors and develops a prognostic algorithm using the predictors, which is a novel result of clinical relevance. The variables identified as important in these models and the optimal cut-points for these variables may prove useful in risk-stratifying premature infants with respiratory failure for future clinical trials. The models may also help in assessment of prognosis, in discussions with parents, and in generating hypotheses that can be tested in clinical trials.
The strengths of this study include the relatively large sample, recruitment from multiple tertiary care NICUs, and prospective data collection by trained observers. The outcomes of death or physiologic BPD at 36 weeks’ post-menstrual age are also relatively well defined. In addition, rather than empirical or expert-opinion derived decision trees, we used variables associated statistically with the outcomes of interest and cut-points for continuous variables which optimized discrimination between those with and without these outcomes. For example, cut-points were identified at 1072 g for birth weight, 90% or more for FiO2, and 2 or more doses of surfactant. The cut-off of 1072g is closer to 1100g rather than 1000g, suggesting that infants who are between 1000g and 1100 g may not be ELBW by definition but may be at similar risk to ELBW infants. These cut-points may prove useful in stratifying infants for future clinical trials, instead of using empirical criteria such as <1000 g or <1250 g birth weight. A limitation is that despite the relatively large sample size for this population, it was not feasible to use a split-half cross-validation approach in which we could develop the model in half of the data set and test it in the other half. Therefore, these models need to be validated using other data sets. Also, it is difficult to distinguish between illness severity and aggressive therapy, as indicators of illness severity (e.g. OI, FiO2) primarily reflect the aggressiveness of therapy rather than the magnitude of underlying lung disease.
Other investigators have developed prediction models for BPD, which have also identified similar variables (lower birth weight or gestational age and increased severity of respiratory illness) as risk factors for BPD.7,8,9 Recently completed trials of iNO in premature infants2-4 indicate that iNO may benefit some premature infants, generally in less ill populations. However, infants evaluated in the current study were smaller and sicker with a very high oxygenation index, and a large proportion either died or developed BPD. Therefore, our models may be more suitable for use in premature infants with severe respiratory failure and may be less suitable for less sick infants. These models are suitable for risk stratification or assessment of prognosis but should not be a basis for decisions regarding withdrawal of support, unless validated on individual center data.
In any prognostic system, certain variables (e.g. pH, PaCO2) are relatively objective while others dependent on clinical examination or clinical judgment (e.g. use of surfactant or high-frequency ventilation) may be subjective, leading to significant inter-center and inter-rater variation. Although the center variable was significantly associated with death and/or BPD by preliminary regression analysis, the center variable was not included for the final analysis, as this limits generalizability of the prediction models, and the interpretation is limited as the clinical practices that lead to center variation are unknown.
The predictor variables that were selected by both regression models and the CART models were very similar, although the exact order in which they were identified was different. Regression analysis and classification tree models in head-to-head comparisons have a comparable performance.10,11,12 Logistic regression is a standard statistical technique in medical literature and is useful in determining the magnitude of the association between each risk factor and the outcome.12 However, estimation of the likelihood of poor outcome for individuals using the logistic regression equation is not practical in the clinical setting. The CART model may be simpler to use for clinicians and ancillary staff, as use of the model involves following a decision tree which provides a qualitative answer (likely or not likely to develop death or BPD).
An in-depth look at the variables selected by CART and by regression analysis provides new data for evaluation in future clinical studies. Birth weight was the variable that was most associated with outcome. This is consistent with literature indicating that birth weight and gestational age perform better for predicting the combined outcome of BPD/death as compared to indices of respiratory failure.13 Male gender was identified as a risk factor for death/BPD, confirming the increased susceptibility of premature male neonates to poor outcomes is well known.14 A higher OI and mean FiO2 are consistent with an increased severity of respiratory failure. A higher peak OI has been associated with death from respiratory failure in preterm infants,13 term infants15 and in older pediatric patients.16 An earlier age at randomization was also associated with worse outcome. It is likely that extremely premature infants with an increased severity of respiratory distress syndrome would have been randomized earlier, and hence, an earlier age of randomization may be consistent with an increased severity of respiratory failure, as are more surfactant doses. It is interesting to note that conventional ventilation (as compared to high frequency ventilation) was associated with a higher risk of death and/or BPD by regression analysis but not by CART analysis. The association of conventional ventilation with death and/or BPD was also identified in the post hoc analyses of the main trial.1 This is consistent with meta-analyses demonstrating that although there is not a convincing benefit to elective high frequency ventilation,17,18 there may possibly be some benefit with high frequency ventilation used for “rescue” of infants with hypoxemic respiratory failure. 19,20
In summary, stepwise regression and CART models identified variables and the optimal cut-points of the variables that are associated with death and/or physiologic BPD in premature infants with severe respiratory failure. These models may prove useful in assessment of prognosis as well as in stratification of infants for future clinical trials. We also observed that the magnitude of initial response to iNO as used in the trial did not correlate with outcome. Future studies are required to identify short-term indicators of response to iNO, if any, that may be associated with long-term benefit and can be used to adjust iNO dosing and duration.
Acknowledgments
Research support:
Supported by grants from the National Institute of Child Health and Human Development (U10 HD34216, U10 HD27853, U10 HD27871, U10 HD40461, U10 HD40689, U10 HD27856, U10 HD27904, U10 HD40498, U10 HD40521, U01 HD36790, U10 HD21385, U10 HD27880, U10 HD27851, and U10 HD 21373) and from the General Clinical Research Centers Program (M01 RR08084, M01 RR06022, M01 RR00750, M01 RR00070, M01 RR00039, and M01 RR00044).
INO Therapeutics provided study gas and support to centers that were not part of the NICHD Neonatal Research Network.
Abbreviations
- ABG
Arterial Blood Gas
- CART
Classification and Regression Tree
- iNO
Inhaled Nitric Oxide
- NICU
Neonatal Intensive Care Unit
Appendix
The following investigators participated in the Preemie Inhaled Nitric Oxide Study: Brown University Women & Infant’s Hospital - William Oh MD, Angelita Hensman BSN RNC, Daniel Gingras RRT; Emory University - Barbara J. Stoll MD, Lucky Jain MD, Ellen Hale RN BS, Irma Seabrook BS RRT-NPS; Indiana University Riley Hospital for Children and Methodist Hospital - Greg Sokol MD, Dianne Lorant MD, Diana Dawn Appel RN BSN, Lucy Miller RN BSN, Dale Chriscinske BS RRT NPS, Jeff Attwood RRT; Northwestern University - Robin Steinhorn MD, Michael Sautel RRT; Stanford University Lucile Salter Packard Children’s Hospital - Krisa Van Meurs MD, Bethany Ball BS CCRC, Dan Proud RCP; University of Alabama at Birmingham University Hospital-UAB - Waldemar A. Carlo MD, Shirley S. Cosby RN BSN, Robert B. Johnson RRT; University of Cincinnati University Hospital, Cincinnati Children’s Hospital Medical Center and Good Samaritan - Jon Fridriksson MD, Barb Warner MD, Marcia Mersmann RN, Barb Alexander RN, Jody Shively RN, Holly Mincey RN, Mary Hoover RRT, Sharon Sapienz RRT, Eric Stephenson RRT; University of California-San Diego UCSD Medical Center and Sharp Mary Birch Hospital for Women - Neil N. Finer MD, Maynard R. Rasmussen MD, Chris Henderson CRTT, Clarence Demetrio RN, Wade Rich RRT-NPS, Christine Joseph, RRT-NPS; University of Florida Wolfson Children’s Hospital at Baptist Medical Center and Shands Jacksonville Medical Center - Mark Hudak MD, Shannon Osbeck RN BSN, Elizabeth Case RN BSN CCRC, Amanda Kellum RRT, Lamont Hogans RRT; University of Rochester Golisano Children’s Hospital at Strong - Carl T. D’Angio MD, Linda Reubens RN, Greg Hutton RRT; University of Texas - Dallas Parkland - Abbot Laptook MD; Susie Madison RN, Gay Hensley RN, Nancy Miller RN, Glenn Metoyer RRT; University of Texas - Houston Memorial Hermann Children’s Hospital - Kathleen Kennedy MD MPH, Georgia McDavid RN, Danny Emerson RRT RCP; Medical College of Wisconsin - Ganesh Konduri MD, Mike Paquette RCP/CRT, Steven Wong RCP/CRT; Wake Forest University Wake Forest University Baptist Medical Center, Forsyth Medical Center and Brenner Children’s Hospital - Judy Aschner MD, T. Michael O’Shea MD MPH, Nancy Peters RN, B.J. Hansell RRT CCRC, Jennifer Griffin RRT RCP, Clay Adams RRT RCP; Wayne State University Hutzel Women’s Hospital & Children’s Hospital of Michigan - Seetha Shankaran MD, Rebecca Bara RN BSN, Geraldine Muran RN BSN, Wonder Weekfall RRT; Yale University New Haven Children’s Hospital Richard A. Ehrenkranz MD, Patricia Gettner RN, Art Caldwell AS RRT.
The members of the NICHD Neonatal Research Steering Committee were: Brown University - William Oh MD, Case Western University - Avroy A. Fanaroff MD, Duke University - Ronald N. Goldberg MD, Emory University - Barbara J. Stoll MD, Indiana University - James A. Lemons MD, Stanford University - David K. Stevenson MD, University of Alabama at Birmingham -Waldemar A. Carlo MD, University of Cincinnati - Edward F. Donovan MD, University of California-San Diego - Neil N. Finer MD, University of Miami - Shahnaz Duara MD, University of Rochester - Dale L. Phelps MD, University of Texas-Dallas - Abbot R. Laptook MD, University of Texas- Houston - Jon E. Tyson MD MPH, Wake Forest University - T. Michael O’Shea MD MPH, Wayne State University - Seetha Shankaran MD, Yale University - Richard A. Ehrenkranz MD, University of Cincinnati - Alan Jobe MD (Chair).
The members of the Data Coordinating Center (RTI International) were: W. Kenneth Poole PhD, Betty Hastings, Carolyn Petrie MS.
The members of National Institute of Child Health and Human Development were: Rosemary D. Higgins MD, Linda L. Wright MD, Elizabeth McClure MEd.
REFERENCES
- 1.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 3.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 4.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 5.Dimitriou G, Greenough A, Kavvadia V, Devane SP, Rennie JM. Outcome predictors in nitric oxide treated preterm infants. Eur J Pediatr. 1999;158:589–591. doi: 10.1007/s004310051153. [DOI] [PubMed] [Google Scholar]
- 6.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 7.Sinkin RA, Cox C, Phelps DL. Predicting risk for bronchopulmonary dysplasia: selection criteria for clinical trials. Pediatrics. 1990;86:728–736. [PubMed] [Google Scholar]
- 8.Ryan SW, Nycyk J, Shaw BN. Prediction of chronic neonatal lung disease on day 4 of life. Eur J Pediatr. 1996;155:668–671. doi: 10.1007/BF01957150. [DOI] [PubMed] [Google Scholar]
- 9.Yoder BA, Anwar MU, Clark RH. Early prediction of neonatal chronic lung disease: a comparison of three scoring methods. Pediatr Pulmonol. 1999;27:388–394. doi: 10.1002/(sici)1099-0496(199906)27:6<388::aid-ppul5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 10.Ambalavanan N, Carlo WA, Shankaran S, Bann CM, Emrich SL, Higgins RD, et al. Predicting outcome of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–2093. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 11.Germanson TP, Lanzino G, Kongable GL, Torner JC, Kassell NF. Risk classification after aneurysmal subarachnoid hemorrhage. Surg Neurol. 1998;49:155–163. doi: 10.1016/s0090-3019(97)00337-6. [DOI] [PubMed] [Google Scholar]
- 12.Werneck GL, de Carvalho DM, Barroso DE, Cook EF, Walker AM. Classification trees and logistic regression applied to prognostic studies: a comparison using meningococcal disease as an example. J Trop Pediatr. 1999;45:248–251. doi: 10.1093/tropej/45.4.248. [DOI] [PubMed] [Google Scholar]
- 13.Subhedar NV, Tan AT, Sweeney EM, Shaw NJ. A comparison of indices of respiratory failure in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 2000;83:F97–F100. doi: 10.1136/fn.83.2.F97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tyson JE, Younes N, Verter J, Wright LL, National Institute of Child Health and Human Development Neonatal Research Network Viability, morbidity, and resource use among newborns of 501- to 800-g birth weight. JAMA. 1996;276:1645–1651. [PubMed] [Google Scholar]
- 15.Kumar D, Super DM, Fajardo RA, Stork EE, Moore JJ, Saker FA. Predicting outcome in neonatal hypoxic respiratory failure with the score for neonatal acute physiology (SNAP) and highest oxygen index (OI) in the first 24 hours of admission. J Perinatol. 2004;24:376–381. doi: 10.1038/sj.jp.7211110. [DOI] [PubMed] [Google Scholar]
- 16.Trachsel D, McCrindle BW, Nakagawa S, Bohn D. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;15(172):206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 17.Thome UH, Carlo WA, Pohlandt F. Ventilation strategies and outcome in randomised trials of high frequency ventilation. Arch Dis Child Fetal Neonatal Ed. 2005;90:F466–F473. doi: 10.1136/adc.2004.068437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson-Smart DJ, Bhuta T, Cools F, Offringa M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. The Cochrane Database of Systematic Reviews. 2003;(4) doi: 10.1002/14651858.CD000104. Art. No.: CD000104. DOI: 10.1002/14651858.CD000104. [DOI] [PubMed] [Google Scholar]
- 19.Keszler M, Donn SM, Bucciarelli RL, Alverson DC, Hart M, Lunyong V, et al. Multicenter controlled trial comparing high-frequency jet ventilation and conventional mechanical ventilation in newborn infants with pulmonary interstitial emphysema. J Pediatr. 1991;119:85–93. doi: 10.1016/s0022-3476(05)81046-7. [DOI] [PubMed] [Google Scholar]
- 20.Joshi VH, Bhuta T. Rescue high frequency jet ventilation versus conventional ventilation for severe pulmonary dysfunction in preterm infants. The Cochrane Database of Systematic Reviews. 2006;(1) doi: 10.1002/14651858.CD000437.pub2. Art. No.: CD000437. DOI: 10.1002/14651858.CD000437.pub2. [DOI] [PubMed] [Google Scholar]