Figure 3.
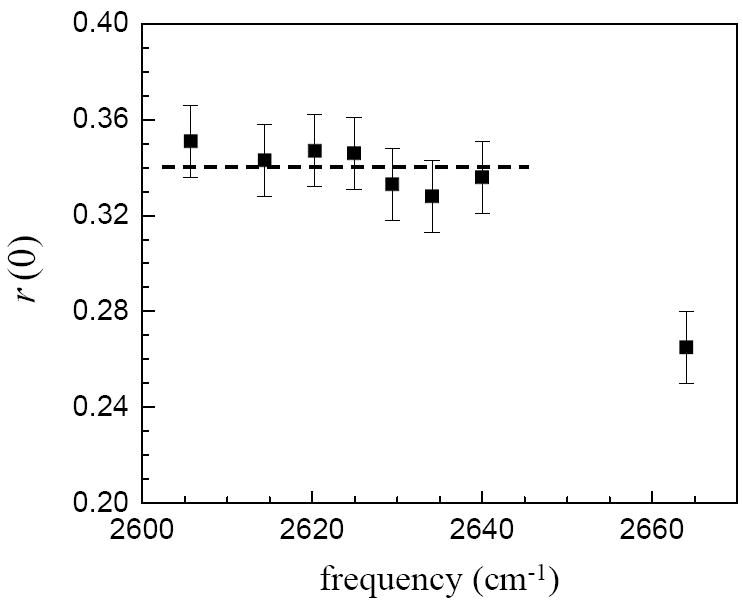
The initial values of the anisotropies following the ultrafast inertial relaxation, r(0), at the center frequencies of the complexes. From left to right the solvents are: 1-pentyne, mesitylene, p-xylene, toluene, benzene, bromobenzene, chlorobenzene, carbon tetrachloride. The dashed line is an aid to the eye drawn at the average value. Within experimental error, all of the complexes have the same amplitude inertial drop, independent of the hydrogen bond strength. Phenol in CCl4, does not form a complex, and it has a substantially different r(0).
