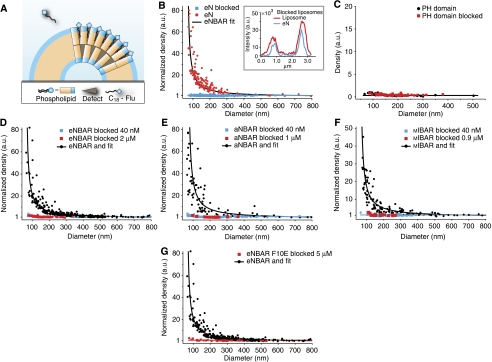
Figure 5.
Membrane curvature sensing by BAR domains is inhibited by blocking the curvature-induced hydrophobic defects of a bilayer. Graphs are measured at 40 nM protein unless otherwise stated. (A) Sketch showing blockage of membrane curvature-induced defects in the bilayer by pre-incubation of liposomes with a small amphiphilic molecule (C18-fluorescein). (B) Blocking of defects impaired membrane curvature sensing mediated by hydrophobic insertion for an AH (eN). Inset: typical line scan showing qualitatively that eN binds to blocked liposomes but without discriminating different diameters. (C) Control with PH domain (1 μM) on normal and blocked liposomes. Both conditions show identical absolute density assuring that blocking with amphiphiles does not cause any general lipid-binding effects. (D–F) Blocking of defects impaired membrane curvature sensing for eNBAR, aNBAR and mIBAR, respectively at both high (2, 1 and 0.9 μM, respectively) and low concentration, further supporting that membrane curvature sensing by BAR domains depends on curvature-induced defects in the bilayer and not charge-mediated interactions. (G) Control with eNBAR F10E at 5 μM also show that the residual binding from a BAR domain is not capable of sensing through charge-mediated interactions.