Figure 5.
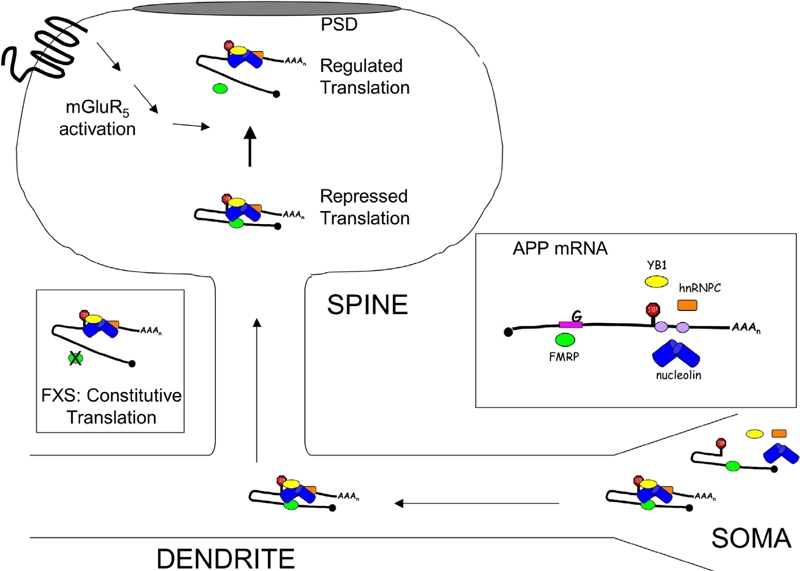
Model of FMRP-Mediated Regulation of APP Translation. APP mRNA is a synaptic target for regulation by FMRP. Through UV crosslinking CLIP assays, we've shown that FMRP binds directly to the coding region of APP mRNA at a guanine-rich region. FMRP also protects a 29-base cis-element in the 3'-UTR from ribonuclease digestion of anti-FMRP immunoprecipitates [1]. RNA binding proteins such as nucleolin, hnRNP C and YB1 bind to cis-elements in the 3'-UTR [43–45]. Nucleolin and YB1 are protein binding factors of FMRP [46–47], which suggests that that protein/protein interactions bring multiple cis-elements in APP mRNA in close proximity to regulate translation (Repressed Translation State). Stimulation of cortical SN with DHPG, a group 1 mGluR agonist, releases FMRP from APP mRNA while increasing APP translation (Regulated Translation State). In the absence of FMRP (fmr-1 KO SN and primary neuronal cells), basal APP levels are increased and nonresponsive to mGluR5 signaling (FXS: Constitutive Translation State). mRNA/protein interactions are likewise important for the movement of APP mRNA from the soma to the dendrites as human APPSwe, which lacks the 3'-UTR, is localized in the soma.
