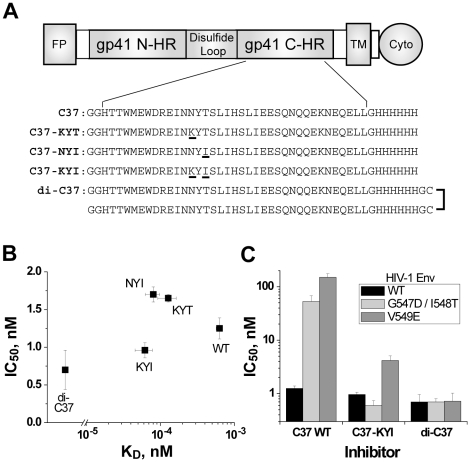
Figure 4. Inhibitory activity of tighter-binding C37 variants.
(A) Schematic of gp41 depicting the N-HR and C-HR segments, fusion peptide (FP), transmembrane (TM), and cytoplasmic (Cyto) domains. The wild type C37 sequence below the diagram is derived from C-HR residues 625–661 of HIV-1HXB2 gp41. The sequences of kinetically restricted variants C37N637K (KYT), C37T639I (NYI), C37N637K/T639I (KYI) are also shown with mutated residues underlined. In the dimeric construct di-C37, two wild type peptides are crosslinked through C-terminal Cys residues. (B) Potency of high affinity C37 variants against wild type HIV-1. IC50 data are plotted as a function of KD for wild type C37 (WT), C37-KYT, C37-NYI, C37-KYI and di-C37. (C) Effect of affinity-disrupting N-HR mutations on C37 potency. IC50 values for C37 WT, C37-KYI, and di-C37 were determined for wild type Env (black), EnvG547D/I548T (light gray) and EnvV549E (dark gray).