Abstract
Interactions between germ cells and surrounding somatic cells are central to ovarian development as well as later function. Disruption of these interactions arising from abnormalities in either cell type can lead to premature ovarian failure (POF). The forkhead transcription factor FOXL2 is a candidate POF factor, and mutations in the FOXL2 gene are associated with syndromic and non-syndromic ovarian failure. Foxl2-deficient mice display major defects in primordial follicle activation with consequent follicle loss, and earlier roles in gonadal development and sex determination have also been suggested. However, despite its importance no data presently exist on its expression in the developing human ovary. Expression of FOXL2 mRNA was demonstrated in the human fetal ovary between 8 and 19 weeks gestation, thus from soon after sex determination to primordial follicle development. Expression in the ovary was higher after 14 weeks than at earlier gestation weeks and was very low in the fetal testis at all ages examined. Immunolocalization revealed FOXL2 expression to be confined to somatic cells, both adjacent to germ cells and those located in the developing ovarian stroma. These cells are the site of action of oocyte-derived activin signalling, but in vitro treatment of human fetal ovaries with activin failed to reveal any regulation of FOXL2 transcription by this pathway. In summary, the expression of FOXL2 in somatic cells of the developing human ovary before and during follicle formation supports a conserved and continuing role for this factor in somatic/germ cell interactions from the earliest stages of human ovarian development.
Keywords: fetal ovary, forkhead, germ cell, primordial follicle, transcription factor
Introduction
Gonadal development is preceded by germ cell formation and migration from the proximal epiblast of the embryo to the urogenital ridge. During and following gonadal colonization, germ cells proliferate: while the majority subsequently undergo apoptosis, some go on to form primordial follicles in conjunction with surrounding somatic cells (Byskov, 1986). While appropriate germ cell development is clearly essential for subsequent fertility, it is evident that the somatic cells of the gonad are critical for the formation of the microenvironment in which the germ cells can mature (McLaren, 1991; De Felici, 2000), known as the germ cell niche. These interactions between germ and somatic cells regulate germ cell proliferation, meiotic entry and arrest and formation of the primordial follicle complement, the latter of which is required in women to ensure several decades of fertile life as well as the endocrine function necessary for puberty, ovulation, fertilization and establishment of pregnancy. This period of development occurs over many weeks in the human fetal ovary with considerable overlap such that the oogonial mitosis continues beyond the onset of meiosis at 9 weeks post coitum (11 weeks gestation) (Gondos et al., 1986; Speed, 1988; Baker and Neal, 1974; Fulton et al., 2005; Bendsen et al., 2006). The less mature germ cells are localized to the periphery of the ovary, with progressively more mature germ cells located deeper in the tissue (Anderson et al., 2007). This spatial and temporal arrangement differs from the rodent where these stages occur in synchrony over 10–12 days, culminating in follicle formation in the immediate neonatal period.
Premature ovarian failure (POF), defined as the onset of the menopause before the age of 40 years, can broadly result from disorders of follicle formation or premature depletion. It is relatively common, affecting 1% of women (Goswami and Conway, 2005). A range of causes of POF have been identified, including genetic, metabolic, autoimmune, infectious and iatrogenic mechanisms; however, many cases are currently idiopathic. Genetic mechanisms include X chromosome abnormalities and mutations in specific autosomal genes, both dominant and recessive: among these dominant phenotypes is the gene encoding the Forkhead box L2 protein (FOXL2). Mutations in FOXL2 result in blepharophimosis/ptosis/epicanthus inversus syndrome (BPES). Type 1 BPES is associated with ovarian failure (although males are fertile) and FOXL2 mutations producing truncated proteins. Type II BPES is a hypomorphic phenotype associated with the production of abnormally long mutant proteins arising from expansions in a polyalanine tract of unknown function (although correlation between genotype and phenotype is imprecise) (Crisponi et al., 2001; De Baere et al., 2001, 2003). FOXL2 is a member of the winged helix/forkhead transcription factor family, of which there are 39 known members in the human and mouse genomes with a variety of functions acting as transcriptional activators and repressors (Carlsson and Mahlapuu, 2002). Consistent with the phenotypes seen in BPES, murine Foxl2 has been localized to Rathke's pouch in the developing pituitary, the developing eyelids and ovarian follicles (Crisponi et al., 2001; Cocquet et al., 2003). Expression in ovarian somatic cells is detectable from early stages of ovarian development through to adulthood (Cocquet et al., 2003), with expression from embryonic day (e)13 in mice (Loffler et al., 2003; Pannetier et al., 2003).
Female mice that are homozygous for targeted disruptions of the Foxl2 gene are infertile, due to a failure of granulosa cells to progress from squamous to cuboidal, preventing the formation of primary follicles (Schmidt et al., 2004; Uda et al., 2004). Defects in primordial follicle formation are also evident (Uda et al., 2004) indicating an earlier onset of critical Foxl2 action. Subsequent studies have indicated that Foxl2 is necessary for gonadal differentiation and that it is one of only a few genes identified as necessary for female gonadal specification (Ottolenghi et al., 2005, 2007).
While FOXL2 is known to be expressed by granulosa cells of the adult human ovary (Crisponi et al., 2001) and is necessary for human ovarian function, it may also be involved in sex determination and normal formation of primordial follicles. We have therefore explored its expression in the developing human ovary up to the time of primordial follicle formation.
Methods
Tissue samples
Human fetal gonads were obtained after medical termination of pregnancy at a range of gestations (8–19 weeks). Gestational age was determined by ultrasound during pregnancy and, for second trimester specimens, confirmed by direct measurement of foot length. Termination of pregnancy was induced by treatment with mifepristone (200 mg orally) followed by misoprostol (200 mg per vaginum every 3 h). None of the terminations was carried out for reasons of fetal abnormality, and all fetuses studied appeared morphologically normal. Collection of fetal samples was approved by the Lothian Research Ethics Committee, and written consent was obtained. Gonads were dissected and snap frozen at −80°C or fixed in Bouin's fluid, transferred into 70% ethanol and processed into paraffin using standard histological methods. Sex of first trimester specimens was determined by detection of SRY in males by PCR (Friel et al., 2002). A total of 25 fetal specimens were used in these studies.
RNA extraction and cDNA synthesis
RNA extraction was performed using RNeasy Micro (1st trimester specimens, mouse ovaries) or Mini (2nd trimester specimens and cultures) Kits (Qiagen, Crawley, UK), and concentration and purity of extracted RNA were assessed using a NanoDrop 1000 (NanoDrop Products, Wilmington, DE, USA). RNA was reverse transcribed with Expand Reverse Transcriptase as described previously (Coutts et al., 2008; gestation analyses) or using the Superscript III First Strand Synthesis Supermix Kit (Invitrogen, Paisley, UK; cultures).
Polymerase chain reaction
Polymerase chain reaction (PCR) was performed using ImmoMix Red (Bioline, London, UK). For PCR amplification, 0.5 µl of each cDNA sample was added to a 25 µl reaction containing 1× ImmoMix Red PCR Master Mix and 500 nM forward and reverse primers suitable for amplifying both the human and mouse genes for FOXL2: F: 5′-TACTCGTACGTGGCGCTCAT-3′, R: 5′-CTCGTTGAGGCTGAGGTTGT-3′ or Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Hartley et al., 2002) as a control. PCR cycling conditions were as follows: 10 min at 95°C; 30 cycles of 30 s at 94°C, 30 s at 53°C, and 30 s at 72°C; 10 min at 72°C. Negative controls included RT- samples and nuclease-free water. PCR products were run on a 2.5% agarose gel with a 100 bp ladder (Promega, Southampton, UK) for confirmation of predicted fragment size (FOXL2:162 bp; GAPDH: 212 bp) and stained with GelRed.
Quantitative RT–PCR
Quantification of FOXL2 mRNA expression relative to that for GAPDH (gestation analyses) or the housekeeping gene RPL32 (F: 5′-CATCTCCTTCTCGGCATCA-3′, R: 5′-AACCCTGTTGTCAATGCCTC-3′; cultures) was measured in duplicate on 1/10 dilutions of each cDNA in 10 µl reactions using the Quantitect SYBRGreen Kit (Qiagen) on the the Roche LightCycler 1.0 instrument (gestation analysis) or PowerSYBR Master Mix on a ABI7900HTFast instrument (both Applied Biosystems, Warrington, UK; cultures) each with appropriate 200 nM forward and reverse primers. LightCycler runs consisted of a hot start at 95°C for 15 min, followed by 45 cycles of 15 s at 95°C, 20 s at 56°C and 20 s at 72°C, with fluorescence detection at the end of each extension step. ABI7900HT runs used the manufacturer's default two-step protocol in standard mode for 40 cycles. On both instruments, melt curve analysis was also performed to confirm specific products and standard curves (using increasing dilutions of second trimester ovary cDNA and P2 mouse cDNA) were performed to confirm efficient amplification of each gene before analysis of all samples using the ΔCt method.
Immunofluorescence
Paraffin-embedded gonads were cut into 5 µm sections and mounted onto electrostatically charged slides (VWR, Leicestershire, UK). After dewaxing and rehydration, antigen retrieval was performed by pressure cooking for 5 min in 0.01 M sodium citrate (pH 6.0). Slides were incubated in 3% hydrogen peroxide in methanol to quench endogenous peroxidases, washed in phosphate-buffered saline (PBS), and blocked in PBS containing 5% Bovine Serum Albumin and 20% Normal Goat Serum (NGS, single immunofluorescence) or 20% normal chicken serum (CS, dual staining). Slides were washed in PBS and incubated with anti-FOXL2 primary antibody (1/500; provided by L. Crisponi; Uda et al., 2004) overnight at 4°C. Slides were washed twice in PBS and incubated with secondary antibody (goat anti-rabbit, 1/200 dilution, Dako, Cambridge, UK). Following two washes in PBS, slides were incubated with Tyramide Cy3 Green (Perkin Elmer Life Sciences, Beaconsfield, Bucks, UK, diluted 1:50) for 10 min at room temperature in a covered slide tray then washed in PBS, and counterstained with propidium iodide for 10 min. For co-localizing FOXL2 and OCT4, after the tyramide step slides were washed in 0.05% PBS Tween (PBST), rinsed in PBS, incubated in EnVision Peroxidase Block (Dako), washed in PBS and blocked again in 20% CS. OCT4 antibody was applied overnight at 4°C. Following PBS washes, slides were incubated with secondary antibody (chicken anti-rabbit for FOXL2, chicken anti-goat for OCT4 (both Santa Cruz Biotechnology, Santa Cruz, CA USA, 1/200 dilution), washed again in PBS then incubated in Tyramide Cy3 Red (TSA Plus Cyanine 3 System, diluted 1:50) for 2 min at room temperature in a covered slide tray. Slides were rinsed in PBST then PBS and counterstained with DAPI (4',6-diamidino-2-phenylindole, Sigma). In each case, glass coverslips were mounted using PermaFluor Aqueous Mounting Medium (Beckman Coulter, High Wycombe, UK), and slides visualized and imaged using an LSM510 meta-Confocal microscope (Carl Zeiss, Welwyn Garden City, Herts, UK). Negative controls were achieved by substituting blocking serum for primary antibodies.
Culture of disaggregated ovaries with activin and follistatin
Human fetal ovaries (17–19 weeks, n = 5) were disaggregated and cultured in serum-free medium exactly as described previously (Coutts et al., 2008). Cultures were performed in the presence or absence of 10 ng/ml human recombinant activin A or 400 ng/ml human recombinant follistatin (both R&D Systems, Abingdon, UK) for 4 or 24 h. After culture, cells were collected and RNA extracted as described above.
Results
FOXL2 mRNA is present in fetal gonads throughout development
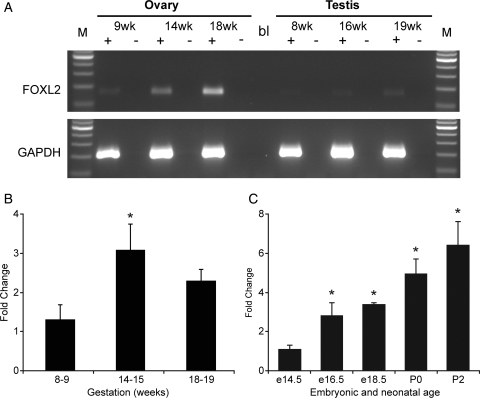
RT–PCR amplification of FOXL2 mRNA revealed it to be expressed in both first and second trimester ovaries and testes (Fig. 1A). Expression in fetal testis was very low at all gestations however, and consistently below the limit of quantification. In contrast, expression in both first and second trimester ovary was readily quantifiable, and showed higher expression in the second trimester than the first with expression at 14–15 weeks being 2.9-fold that at 8–9 weeks (P < 0.05, Fig. 1B). There was no difference in expression between early second trimester (14–15 weeks, germ cell proliferation and entry into meiosis) and later specimens (18–19 weeks: onset of primordial follicle formation).
Figure 1.
Detection and quantification of FOXL2 gene expression in the human fetal gonad. (A) Expression of FOXL2 and GAPDH mRNA in the human fetal ovary and testis. Samples at indicated gestational age were analysed by RT–PCR. ‘+’ and ‘−’indicate the presence or absence of reverse transcriptase during cDNA synthesis. M: 100 bp ladder, bl: water blank in PCR reaction. (B) Quantitative PCR measurement of FOXL2 expression in the human fetal ovary at gestations of 8–9 weeks, 14–15 and 18–19 weeks as indicated, n = 4–5 per group. (C) Quantitative PCR measurement of Foxl2 expression in embryonic and post-natal mouse ovary at ages indicated, n = 3-5 per group. Both human and mouse data were analysed by ANOVA with Duncan's post hoc test using earliest gestation as comparator, *P < 0.05.
Expression of the paralogous gene Foxl2, in the embryonic and neonatal mouse ovary over the equivalent period of development was also investigated. This demonstrated a progressive increase in Foxl2 expression between embryonic day 14.5 and post-natal day 2, with an overall increase of 6.4-fold (P < 0.001, Fig. 1C).
FOXL2 is expressed by somatic cells of the human fetal ovary
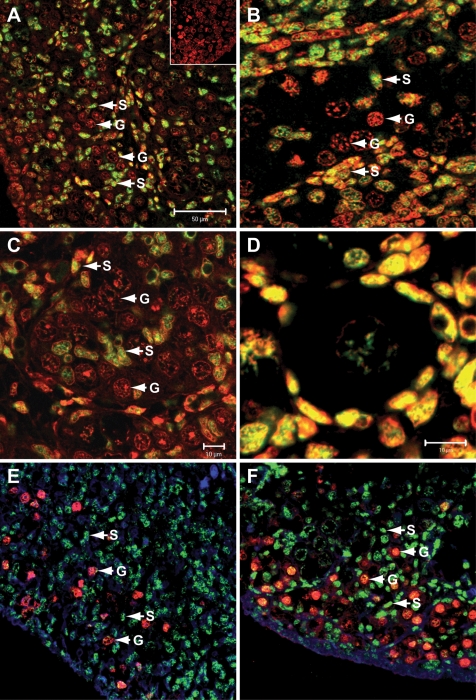
Immunofluorescent staining localized FOXL2 expression to somatic cells of the human fetal ovary in both the first and second trimesters (Fig. 2). Expression was predominantly nuclear, in keeping with FOXL2 being a transcription factor. FOXL2 was notably present in the somatic cell streams surrounding germ cell clusters (Fig. 2A–C) but it was also expressed by somatic cells intermingled with germ cells (Fig. 2B and C): this population will become the pre-granulosa cells of primordial follicles. These cells also displayed intense staining by immunofluorescence after primordial follicle formation (Fig. 2D, 19 weeks gestation). Somatic cell specificity was confirmed by the absence of colocalization of FOXL2 with the germ cell-specific marker OCT4 (Fig. 2E, 9 weeks gestation; F, 19 weeks): OCT4 labels all germ cells in the first trimester and less mature germ cells, located at the periphery of the ovary, in the second trimester (Anderson et al., 2007). FOXL2 expression was not detected in the fetal testis between 8 and 19 weeks gestation (not shown).
Figure 2.
Immunolocalization of FOXL2 in the human fetal ovary. A and B, 14 weeks; C and D, 19 weeks. Positive immunofluorescence for FOXL2 is green, counterstain is propidium iodide (red), hence nuclear FOXL2 is yellow. Inset in (A) is negative control. (E, F) Double immunofluorescence for FOXL2 (green) and OCT4 (red) in human fetal ovary showing absence of colocalization, with FOXL2 expressed by somatic cells and OCT4 by germ cells. E, 9 weeks gestation; F, 19 weeks. Blue nuclear stain is DAPI. G: germ cell, S: somatic cell. Scale bars are 50 µm (A, applies to E and F) or 10 µm (B, C, D).
Activin does not regulate FOXL2 expression
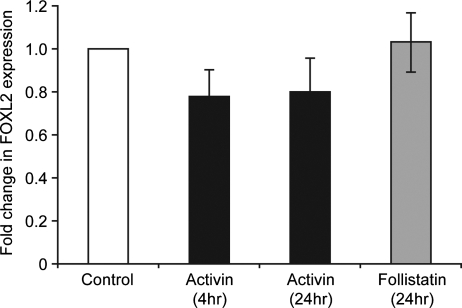
The somatic cells shown here to express FOXL2 are the same population that are the site of action of activin within the second trimester ovary (Coutts et al., 2008). The possible regulation of FOXL2 expression by activin was therefore investigated. Treatment of disaggregated ovarian tissue with activin A (10 ng/ml, n = 5) for 4 and 24 h resulted in a small but non-significant reduction in expression of FOXL2 (78 ± 12% of control at 4 h, Fig. 3). Treatment with the activin antagonist follistatin (400 ng/ml, n = 5, 24 h) had no effect on FOXL2 expression (103 ± 14% of control).
Figure 3.
Expression of FOXL2 in disaggregated human fetal ovary (17–19 weeks gestation) cultured with activin A (10 ng/ml) for 4 or 24 h as indicated, or with follistatin (400 ng/ml, 24 h) versus untreated control cultures. Mean ± SEM, n = 5.
Discussion
An increasing number of genes associated with POF are being identified (Goswami and Conway, 2005), largely based on laboratory studies investigating the key regulatory factors and pathways in ovarian development (Matzuk and Lamb, 2002; Pangas and Rajkovic, 2006). One such is the forkhead transcription factor FOXL2, mutations in which are associated with POF and BPES (Crisponi et al., 2001). Ovarian biopsies in BPES type 1 show unstimulated primordial follicles (‘resistant ovary’), or no follicles (Fraser et al., 1988; Nicolino et al., 1995). Non-syndromic POF has also been described in association with a polyalanine deletion in FOXL2 (Gersak et al., 2004). Mouse models with targeted disruptions of the Foxl2 locus have been generated (Schmidt et al., 2004; Uda et al., 2004), indicating a major defect in the activation of primordial follicles due to a failure of granulosa cells to progress from squamous to cuboidal. Although the ovaries of Foxl2 null mice have a normal oocyte and follicle number in the neonatal period (Schmidt et al., 2004; Uda et al., 2004), defects in primordial follicle formation are evident (Uda et al., 2004). Insect FoxL has also been implicated in oocyte production (Hansen et al., 2007). Here we have demonstrated the expression of FOXL2 in the developing human ovary, by somatic but not germ cells, throughout the stages of ovarian development from germ cell proliferation in the first trimester through the onset of meiosis to primordial follicle formation.
Expression of FOXL2 mRNA was readily detectable in the earliest gestation specimens investigated (8 weeks gestation, 6 post-ovulatory weeks). It therefore remains to be determined how early FOXL2 expression commences in the human ovary, but it clearly precedes the onset of meiosis. In the mouse, expression is first detected at e13 (Loffler et al., 2003; Pannetier et al., 2003), coincident with the onset of meiosis. Expression increased approximately 3-fold between 8–9 and 14–15 weeks gestation, but there was no further increase at weeks 18–19, at which stage primordial follicle formation has started. This therefore differs from the progressive rise demonstrated in the mouse, and also in other factors associated with primordial follicle formation reported by ourselves and other groups (Bayne et al., 2004; Fowler et al., 2009). An additional key role for FOXL2 in earlier developmental processes than follicle formation has been suggested (Ottolenghi et al., 2007; Garcia-Ortiz et al., 2009). This was based on the demonstration that mice with targeted deletions of Foxl2 show only subtle defects in primordial follicle formation, whilst those doubly deficient for both Foxl2 and Wnt4 show a severe ovarian phenotype at birth (i.e. prior to follicle formation) distinct from the Wnt4-only null (Ottolenghi et al., 2007). These double-null ovaries showed evidence of sex reversal with tubule formation and markedly increased expression of the male-specific gene Sox9, normally expressed only in Sertoli cells. Recent data indicate that Sox9 actively represses Foxl2 expression in Sertoli cells (Wilhelm et al., 2009). The goat polled/intersex syndrome (PIS), characterized by the absence of horns (polledness) and XX female to male sex reversal (Vaiman et al., 1996), is also associated with mutations in Foxl2 (Pailhoux et al., 2001). These and the present data are consistent with a sex-determining role for Foxl2 in large mammals as well as rodents.
A low level of expression of FOXL2 mRNA was detected in the human testis in both the first and second trimester. This is in agreement with detection of Foxl2 mRNA in the developing goat testis and in the mouse testis between e13.5 and e15.5 (Pailhoux et al., 2001; Loffler et al., 2003). We were unable to detect FOXL2 protein in the testis by immunofluorescence, thus the low level of expression may not be of functional importance, in keeping with the preserved fertility of men with BPES and FOXL2 mutations (Crisponi et al., 2001; De Baere et al., 2003).
Immunofluorescence demonstrated that expression of FOXL2 was confined to the somatic cells of the developing ovary. Foxl2 has been localized previously to the somatic cells of the neonatal and adult rodent ovary (Cocquet et al., 2003; Loffler et al., 2003; Pisarska et al., 2004), and to follicles of the adult human ovary (Crisponi et al., 2001), thus this selective pattern of expression is conserved. A search for downstream targets of Foxl2 identified that expression of the steroidogenic acute regulatory (StaR) gene was directly repressed by Foxl2 in the adult mouse ovary (Pisarska et al., 2004) but it is uncertain whether this is involved in the primordial follicle activation seen in Foxl2-deficient mice. In this study, FOXL2 was found to be expressed by both main populations of somatic cells within the developing ovary in the second trimester. One population is intermingled with the germ cells within germ cell cysts or nests: these are syncitial groups of synchronously dividing germ cells, which subsequently break down yielding individual germ cells that are either lost through apoptosis or other pathways, or go on to form the oocytes within primordial follicles. This population of somatic cells is therefore intimately associated with germ cells across a range of developmental stages, regulation of which is essential for the formation of the primordial follicle complement. The second population of somatic cells constitute what have been termed the ‘cell streams’ (McNatty et al., 2000) which make up a meshwork between and around the germ cell nests, but are separated from them by a basement membrane (Sawyer et al., 2002). This is incomplete at the periphery, where these somatic cells intermingle with the less mature germ cells which are at a similar stage of development to those in the first trimester, on the basis of expression of the pluripotency-associated factor OCT4 (Anderson et al., 2007). The widespread expression of FOXL2 by both populations of somatic cells is consistent with multiple roles during ovarian development, both related to sex determination possibly in conjunction with WNT4 and in germ cell/somatic cell interactions leading to primordial follicle formation.
There is emerging evidence that the transforming growth factor TGFβ family member activin A is a key regulator of ovarian development. Activin βA is specifically expressed by more mature germ cells within the human ovary in the second trimester (Martins da Silva et al., 2004), acting on surrounding somatic cells (Coutts et al., 2008) which are here shown to also be the site of expression of FOXL2. In both humans (in vitro) and mice (in vivo), activin treatment of the fetal ovary increases the number of germ cells and, in mice, of primordial follicles (Martins da Silva et al., 2004; Bristol-Gould et al., 2006). There are data from a number of developmental systems of interactions between TGFβ members and forkhead transcription factors, including mutual regulation of expression (Zhou et al., 2002; Sommer et al., 2006) and interaction with Smad signalling pathways (Attisano et al., 2001), also demonstrated for Foxl2 (Ellsworth et al., 2003; Blount et al., 2009). The present data suggest that activin does not directly regulate FOXL2 expression in the human, but it remains possible that FOXL2 and activin-regulated Smad signalling interact to regulate target gene expression in fetal ovarian somatic cells. Additionally it is possible that other oocyte-expressed members of the TGFβ family such as growth and differentiation factor GDF9 or bone morphogenic protein BMP15 which are known to exert effects on ovarian somatic cells (Li et al., 2008) might interact with FOXL2.
In conclusion, FOXL2 is expressed in the somatic cells of the human ovary from early in development. Only very low levels of expression were found in the testis. Expression precedes the onset of meiosis and is consistent with multiple roles for FOXL2 in ovarian function, possibly including sex determination as well as follicle growth.
Funding
This work was supported by the Medical Research Council (WBS U.1276.00.002.00001.01).
Acknowledgements
We are grateful to Anne Saunderson, Joan Creiger and the staff of the Bruntsfield Suite, Royal Infirmary of Edinburgh, for patient recruitment. We are also grateful to Dr. L. Crisponi for the generous gift of FOXL2 antibody.
References
- Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PTK. Conserved and divergent patterns of gene expression in female and male germ cells during development of the human fetal gonad. BMC Dev Biol. 2007;7:136–145. doi: 10.1186/1471-213X-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L, Silvestri C, Izzi L, Labbe E. The transcriptional role of Smads and FAST (FoxH1) in TGFbeta and activin signalling. Mol Cell Endocrinol. 2001;180:3–11. doi: 10.1016/s0303-7207(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Baker TG, Neal P. Oogenesis in human fetal ovaries maintained in organ culture. J Anat. 1974;117:591–604. [PMC free article] [PubMed] [Google Scholar]
- Bayne RA, Martins Da Silva SJ, Anderson RA. Increased expression of the FIGLA transcription factor is associated with primordial follicle formation in the human fetal ovary. Mol Hum Reprod. 2004;10:373–381. doi: 10.1093/molehr/gah056. [DOI] [PubMed] [Google Scholar]
- Bendsen E, Byskov AG, Andersen CY, Westergaard LG. Number of germ cells and somatic cells in human fetal ovaries during the first weeks after sex differentiation. Hum Reprod. 2006;21:30–35. doi: 10.1093/humrep/dei280. [DOI] [PubMed] [Google Scholar]
- Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem. 2009;284:7631–7645. doi: 10.1074/jbc.M806676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298:132–148. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Byskov AG. Differentiation of mammalian embryonic gonad. Physiol Revs. 1986;66:71–117. doi: 10.1152/physrev.1986.66.1.71. [DOI] [PubMed] [Google Scholar]
- Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002;250:1–23. doi: 10.1006/dbio.2002.0780. [DOI] [PubMed] [Google Scholar]
- Cocquet J, De Baere E, Gareil M, Pannetier M, Xia X, Fellous M, Veitia RA. Structure, evolution and expression of the FOXL2 transcription unit. Cytogenet Genome Res. 2003;101:206–211. doi: 10.1159/000074338. [DOI] [PubMed] [Google Scholar]
- Coutts SM, Childs AJ, Fulton N, Collins CS, Bayne RAL, McNeilly AS, Anderson RA. Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Devel Biol. 2008;314:189–199. doi: 10.1016/j.ydbio.2007.11.026. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- De Baere E, Dixon MJ, Small KW, Jabs EW, Leroy BP, Devriendt K, Gillerot Y, Mortier G, Meire F, Van Maldergem L, et al. Spectrum of FOXL2 gene mutations in blepharophimosis-ptosis-epicanthus inversus (BPES) families demonstrates a genotype–phenotype correlation. Hum Mol Genet. 2001;10:1591–1600. doi: 10.1093/hmg/10.15.1591. [DOI] [PubMed] [Google Scholar]
- De Baere E, Beysen D, Oley C, Lorenz B, Cocquet J, De Sutter P, Devriendt K, Dixon M, Fellous M, Fryns JP, et al. FOXL2 and BPES: mutational hotspots, phenotypic variability, and revision of the genotype-phenotype correlation. Am J Hum Genet. 2003;72:478–487. doi: 10.1086/346118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felici M. Regulation of primordial germ cell development in the mouse. Int J Dev Biol. 2000;44:575–580. [PubMed] [Google Scholar]
- Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM. The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol. 2003;206:93–111. doi: 10.1016/s0303-7207(03)00235-1. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Flannigan S, Mathers A, Gillanders K, Lea RG, Wood MJ, Maheshwari A, Bhattacharya S, Collie-Duguid ES, Baker PJ, et al. Gene expression analysis of human fetal ovarian primordial follicle formation. J Clin Endocrinol Metab. 2009;94:1427–1435. doi: 10.1210/jc.2008-2619. [DOI] [PubMed] [Google Scholar]
- Fraser IS, Shearman RP, Smith A, Russell P. An association among blepharophimosis, resistant ovary syndrome, and true premature menopause. Fertil Steril. 1988;50:747–751. doi: 10.1016/s0015-0282(16)60309-6. [DOI] [PubMed] [Google Scholar]
- Friel A, Houghton JA, Glennon M, Lavery R, Smith T, Nolan A, Maher M. A preliminary report on the implication of RT-PCR detection of DAZ, RBMY1, USP9Y and Protamine-2 mRNA in testicular biopsy samples from azoospermic men. Int J Androl. 2002;25:59–64. doi: 10.1046/j.1365-2605.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- Fulton N, Martins da Silva SJ, Bayne RAL, Anderson RA. Germ cell proliferation and apoptosis in the developing human ovary. J Clin Endocrinol Metab. 2005;90:4664–4670. doi: 10.1210/jc.2005-0219. [DOI] [PubMed] [Google Scholar]
- Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, et al. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev Biol. 2009;9:36. doi: 10.1186/1471-213X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersak K, Harris SE, Smale WJ, Shelling AN. A novel 30 bp deletion in the FOXL2 gene in a phenotypically normal woman with primary amenorrhoea: case report. Hum Reprod. 2004;19:2767–2770. doi: 10.1093/humrep/deh496. [DOI] [PubMed] [Google Scholar]
- Gondos B, Westergaard L, Byskov AG. Initiation of oogenesis in the human fetal ovary: ultrastructural and squash preparation study. Am J Obstet Gynaecol. 1986;155:189–195. doi: 10.1016/0002-9378(86)90109-2. [DOI] [PubMed] [Google Scholar]
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Sieglaff DH, Munro JB, Shiao SH, Cruz J, Lee IW, Heraty JM, Raikhel AS. Forkhead transcription factors regulate mosquito reproduction. Insect Biochem Mol Biol. 2007;37:985–997. doi: 10.1016/j.ibmb.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley PS, Bayne RAL, Robinson LLL, Fulton N, Anderson RA. Developmental changes in expression of myeloid cell leukaemia-1 in human germ cells during oogenesis and early folliculogenesis. J Clin Endocrinol Metab. 2002;87:3417–3427. doi: 10.1210/jcem.87.7.8644. [DOI] [PubMed] [Google Scholar]
- Li Q, McKenzie LJ, Matzuk MM. Revisiting oocyte-somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol Hum Reprod. 2008;14:673–678. doi: 10.1093/molehr/gan064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–3243. doi: 10.1210/en.2002-0095. [DOI] [PubMed] [Google Scholar]
- Martins da Silva SJ, Bayne RAL, Cambray N, Hartley PS, McNeilly AS, Anderson RA. Expression of activin subunits and receptors in the developing human ovary: activin A promotes germ cell survival and proliferation prior to primordial follicle formation. Dev Biol. 2004;266:334–345. doi: 10.1016/j.ydbio.2003.10.030. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4(Suppl.):s41–s49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- McLaren A. Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays. 1991;13:151–156. doi: 10.1002/bies.950130402. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Fidler AE, Juengel JL, Quirke LD, Smith PR, Heath DA, Lundy T, O'Connell A, Tisdall DJ. Growth and paracrine factors regulating follicle formation and cellular function. Mol Cell Endo. 2000;163:11–20. doi: 10.1016/s0303-7207(99)00235-x. [DOI] [PubMed] [Google Scholar]
- Nicolino M, Bost M, David M, Chaussain JL. Familial blepharophimosis: an uncommon marker of ovarian dysgenesis. J Pediatr Endocrinol Metab. 1995;8:127–133. doi: 10.1515/jpem.1995.8.2.127. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- Pailhoux E, Vigier B, Chaffaux S, Servel N, Taourit S, Furet JP, Fellous M, Grosclaude F, Cribiu EP, Cotinot C, Vaiman DA. 11.7-kb deletion triggers intersexuality and polledness in goats. Nat Genet. 2001;29:453–458. doi: 10.1038/ng769. [DOI] [PubMed] [Google Scholar]
- Pangas SA, Rajkovic A. Transcriptional regulation of early oogenesis: in search of masters. Hum Reprod Update. 2006;12:65–76. doi: 10.1093/humupd/dmi033. [DOI] [PubMed] [Google Scholar]
- Pannetier M, Servel N, Cocquet J, Besnard N, Cotinot C, Pailhoux E. Expression studies of the PIS-regulated genes suggest different mechanisms of sex determination within mammals. Cytogenet Genome Res. 2003;101:199–205. doi: 10.1159/000074337. [DOI] [PubMed] [Google Scholar]
- Pisarska MD, Bae J, Klein C, Hsueh AJ. Forkhead l2 is expressed in the ovary and represses the promoter activity of the steroidogenic acute regulatory gene. Endocrinology. 2004;145:3424–3433. doi: 10.1210/en.2003-1141. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Sommer P, Napier HR, Hogan BL, Kidson SH. Identification of Tgf beta1i4 as a downstream target of Foxc1. Dev Growth Differ. 2006;48:297–308. doi: 10.1111/j.1440-169X.2006.00866.x. [DOI] [PubMed] [Google Scholar]
- Speed RM. The possible role of meiotic pairing anomalies in the atresia of human fetal oocytes. Hum Genet. 1988;78:260–266. doi: 10.1007/BF00291673. [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Vaiman D, Koutita O, Oustry A, Elsen JM, Manfredi E, Fellous M, Cribiu EP. Genetic mapping of the autosomal region involved in XX sex-reversal and horn development in goats. Mamm Genome. 1996;7:133–137. doi: 10.1007/s003359900033. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Washburn LL, Truong V, Fellous M, Eicher EM, Koopman P. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech Dev. 2009;126:324–336. doi: 10.1016/j.mod.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kato H, Asanoma K, Kondo H, Arima T, Kato K, Matsuda T, Wake N. Identification of FOXC1 as a TGF-beta1 responsive gene and its involvement in negative regulation of cell growth. Genomics. 2002;80:465–472. [PubMed] [Google Scholar]