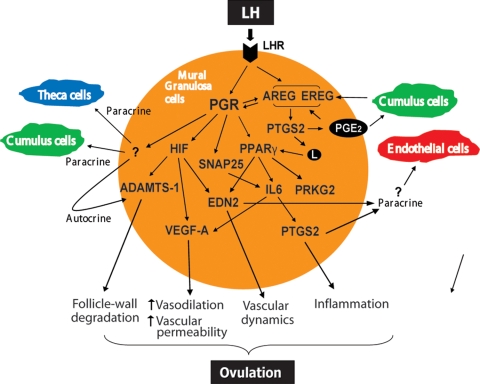
Figure 1:
An emerging blueprint of PGR-regulated gene networks that control ovulation in the mouse. The schematic describes the PGR-regulated factors that are discussed in this review. The PGR, expressed in mural granulosa cells of pre-ovulatory follicles, regulates downstream genes such as peroxisome proliferator-activated receptors (PPARγ) and HIFs, which are important mediators of its function during ovulation. These genes in turn regulate the production of critical factors that are secreted and act on various ovarian cell types, such as cumulus granulosa cells, theca cells and vascular endothelial cells. Both PPARγ and HIFs control the production of endothelin (EDN2), which likely contributes to the follicle-wall degradation by influencing the dynamic changes in the vasculature during ovulation. PPARγ also controls the synthesis of IL6, which acts by regulating VEGF-A, which enhances vascular permeability, and PTGS2, which generates inflammation-inducing fatty-acid metabolites. The fatty-acid metabolites produced by PTGS2 promote ovulation by acting as agonists of PPARγ, thereby forming a classical regulatory loop. SNAP25, another PGR-regulated gene, may also participate in the ovulatory process by regulating the release of cytokines, including IL6, from granulosa cells. PGR also mediates the induction of the EGF-like growth factors, such as AREG and EREG, in granulosa cells. These factors in turn induce PTGS2, which generates prostaglandin E2 (PGE2). AREG and PGE2 can then act on cumulus cells, via paracrine mechanisms, to induce AREG and PTGS2. Since ovulation also involves physical degradation of the follicular wall, the actions of proteases, such as ADAMTS-1, are essential during this process. The coordinated actions of the PGR-regulated pathways eventually bring about the breakdown of the pre-ovulatory follicle, releasing the oocyte. PRKG2, cyclic-GMP-dependent protein kinase; L, fatty-acid metabolites.