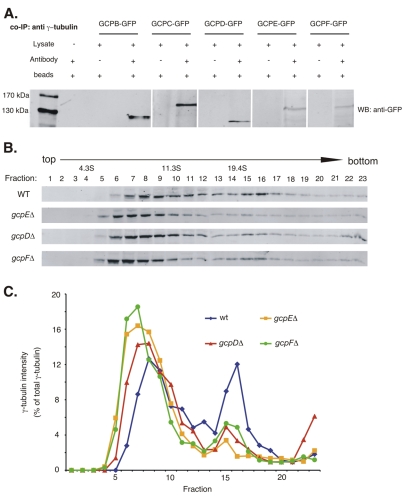
Fig. 2.
Co-immunoprecipitation of putative γ-tubulin complex proteins with γ-tubulin (A) and size distribution of γ-tubulin complexes as determined by sucrose-density centrifugation (B,C). (A) Leftmost lane shows protein size standards. The remaining lanes are from western blots using anti-GFP. For each GCP-GFP fusion strain the left lane shows a mock immunoprecipitation in which the anti-γ-tubulin antibody was omitted. The right lane shows an immunoprecipitation with the anti-γ-tubulin antibody. The anti-GFP recognizes a band of the expected size in each case, indicating that GCPB-GCPF co-immunoprecipitate with γ-tubulin. (B) One set of representative results of western blots, using an anti-γ-tubulin antibody as a probe, of sucrose-density gradients of cytoplasmic extracts of strains wild-type for the GCP-encoding genes (WT) or carrying gcpD, gcpE and gcpF deletions. (C) Plot of the intensity of the γ-tubulin signal for each fraction from the gradients. For each strain, the signal intensity of each fraction is expressed as the percentage of the total γ-tubulin in the sample. In the wild-type strain γ-tubulin is present in a broad peak between approximately 7S and 14S and in a sharper peak at approximately 21S. In the deletion strains, the higher mass peak is greatly diminished and the higher and lower mass peaks are shifted slightly to the left (lower mass).