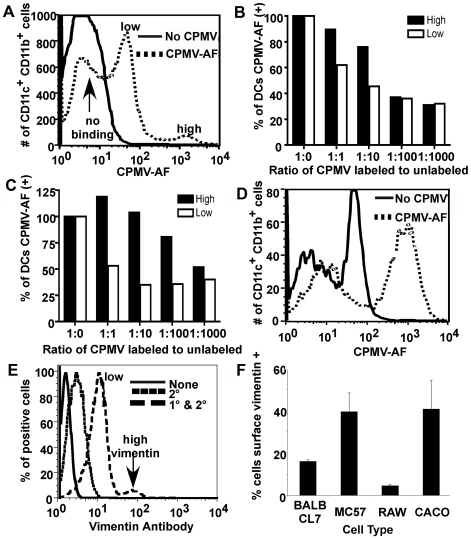
Figure 2. Binding and uptake of CPMV particles by imDCs.
(A, B and C) CPMV binding. (A) FACS analysis of a culture of ten days differentiated bone marrow cells. Cells were incubated on ice for 3 hours with CPMV-AF488 (dashed line), washed and stained with APC-CD11c and PE-CD11b antibody markers to identify imDCs. PBS was added to the control cells (solid line). (B and C) CPMV competition assay. After labeling with APC-CD11c and PE-CD11b markers 1×106 cells (B) or 5×105 cells (C) per well were incubated on ice with different amounts of unlabeled CPMV (0 µg, 1 µg, 10 µg, 100 µg and 1000 µg). After washing, 1 µg of CPMV-AF488 was added to each well generating a ratio of 1∶0, 1∶1, 1∶10, 1∶100 and 1∶1000 of CPMV-AF488 to unlabeled CPMV. The black columns represent the high binding imDCs and the white columns represent the low binding imDCs. (D) FACS analysis of CPMV uptake by imDCs. Cells were incubated for two hours with CPMV-AF488 (dashed line) at 37°C and 5% CO2, washed and stained with APC-CD11c and PE-CD11b antibodies and analyzed by FACS to quantify the internalization of CPMV particles into CD11c+/CD11b+ DCs. PBS was added to the control cells (solid line). (E and F) FACS analysis of surface vimentin expression on MC57 (E, F), BalbCL7, RAW macrophages, and Caco cells (F). Cells were incubated one hour, 4°C with anti-vimentin V4630 antibody, then one hour, 4°C with secondary AF647 antibody.