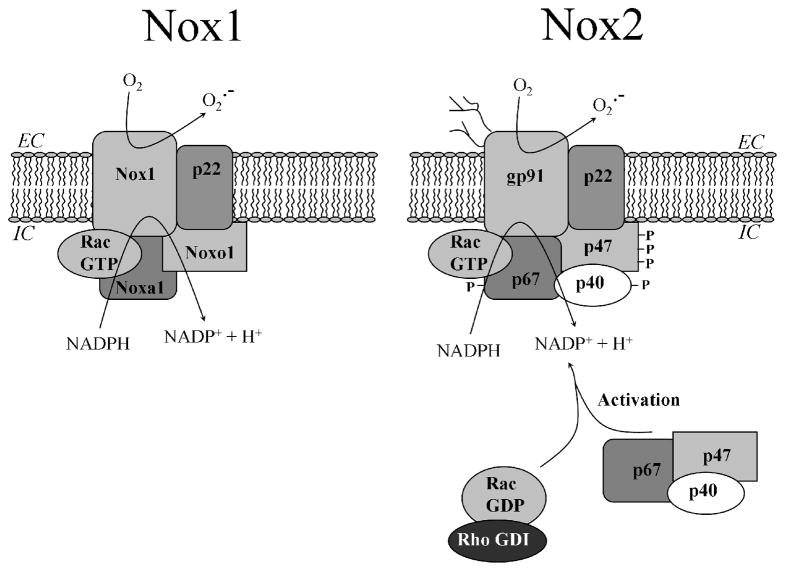
Figure 2. Activated complexes of multi-component Nox1- and Nox2-based NADPH oxidases.
The Nox1 and Nox2(gp91phox) flavocytochromes form heterodimeric complexes with a common p22phox chain. Both oxidases are regulated by homologous organizer (Noxo1 or p47phox) and activator (Noxa1 or p67phox) proteins, and require GTP-bound Rac. The cytosolic subunits of Nox2 (p47phox, p67phox and p40phox) are preassembled in the cytosol and translocate to the flavocytochrome upon activation. In resting cells, Rac is found in a GDP-bound state stabilized by RhoGDI. When activated, both oxidases produce superoxide anions. Nox1 is localized to the plasma membrane of colon epithelial cells and produces superoxide into the extracellular space, whereas Nox2 is assembled and activated on phagosomes of phagocytic cells.