Figure 2.
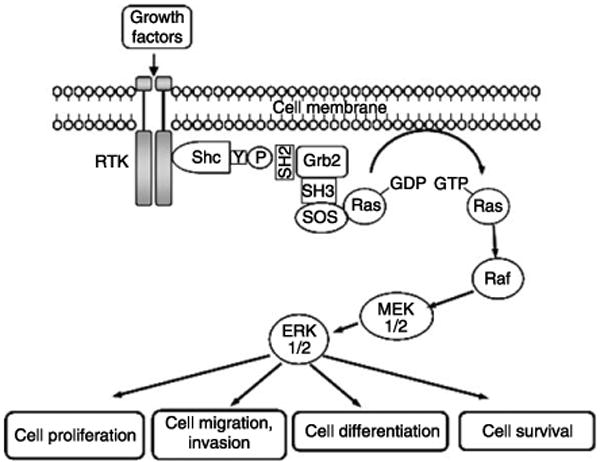
A schematic representation of Shc proteins activating the Ras and MAP kinase cascade. Upon stimulation by growth factors, RTK undergoes dimerization and tyrosine phosphorylation. Subsequently, Shc proteins are recruited and phosphorylated on tyrosine residue via forming a complex with RTK through the SH2 and/or PTB domain of Shc. The phosphorylated Shc proteins then associate with Grb2 adaptor protein through its tyrosine phosphorylation site to the SH2 domain of Grb2; the latter is constitutively complexed with SOS through its SH3 domains. These events result in the translocation of SOS to the plasma membrane and subsequently activate membrane-bound Ras in the exchange of GDP for GTP and trigger the activation of MAP kinase cascade, resulting in cell proliferation, differentiation, migration, invasion, and survival.
