Figure 5.
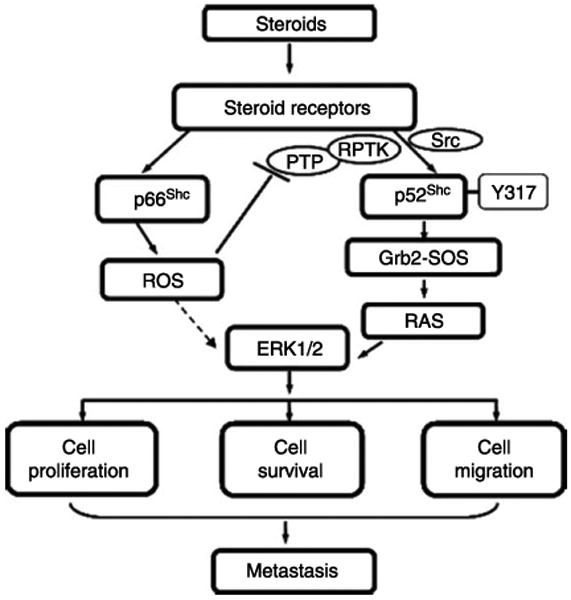
A proposed scheme of steroid-regulated cancer progression via Shc proteins. Upon steroid activation, p52Shc undergoes phosphorylation at Y317 and promotes tumor growth at least in part via transducing signals through the Grb2–Ras–MAPK pathway. Like p52Shc, p66Shc protein also promotes tumor progression, nevertheless, via mediating oxidative stress signals through the generation of ROS. Upon stimulation by steroids, p66Shc is translocated to mitochondria via Ser-36 phosphorylation-independent manner resulting in the generation of ROS. ROS may then inhibit PTP, resulting in RPTK activation, ERK/MAPK activation, and promote cell proliferation, survival, and migration, which collectively lead to metastasis.
