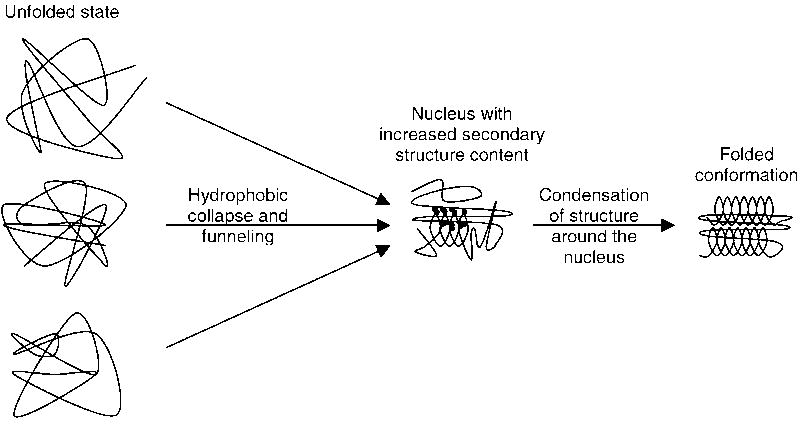
Fig. 4.
Synthesis of the findings of the high resolution analysis of the folding pathways for at least ten of the 15 proteins into a unified nucleation–condensation mechanism for two-state folding of small globular proteins: Early folding events are largely connected with a size decrease and funneling of different conformations into a folding nucleus which often has a larger fraction of secondary structure forming residues. The nucleus then catalyzes folding by enabling the condensation of further structure around it. Since the nucleus has only some degree of stability if it contains a sufficient number of correct secondary and tertiary structure interactions, it can very efficiently prevent a high degree of misfolding in the later stages of folding.