Table 1.
Catalytic Asymmetric C-H Diamination of Terminal Olefinsa
| Entry | Substrate | Productb | Yield (%)c | ee (%)d |
|---|---|---|---|---|
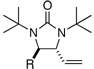 |
||||
| 1 | R = Et | 50 | 90 | |
| 2 | R =nC5H11 | 71 | 91 | |
| 3 | R = CH2Ph | 67 | 93e,f | |
| 4 | R = i-Pr | 51 | 94g | |
| 5 | R = Ph | 80 | 90e | |
| 6 | 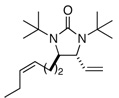 |
80 | 92 | |
| 7 | 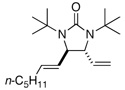 |
81 | 90g | |
| 8 | 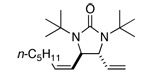 |
69 | 89 | |
| 9 | 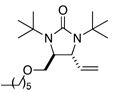 |
85 | 91 | |
| 10 | 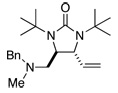 |
67 | 92 | |
| 11 | 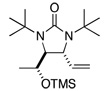 |
70 | 97:3h,i (dr) | |
| 12 | 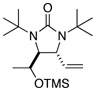 |
66 | 94:6h (dr) |
All reactions were carried out with olefin (0.80 mmol), 2 (2.0 mmol, 2.5 equiv), Pd2(dba)3 (0.04 mmol), and ligand L2 (0.176 mmol) at 65 °C for 6 h.
The structures represent only proposed absolute configurations by analogy.
Isolated yield based on olefin.
The ee was determined by chiral-GC (Chiraldex B-DM column) unless otherwise stated.
The ee was determined by chiral HPLC (Chiralpak AD column) after the removal of t-butyl groups.
The (R,R) configuration was determined by comparing the optical rotation with the reported one (see ref. 9a).
The ee was determined by chiral-GC (Chiraldex B-DM column) after the removal of t-butyl groups.
The ratio was determined by achiral GC (VA-5MS column).
The (2R,3S,4R) configuration was determined by the X-ray structure of diamination product after the removal of TMS group.
