Figure 3.
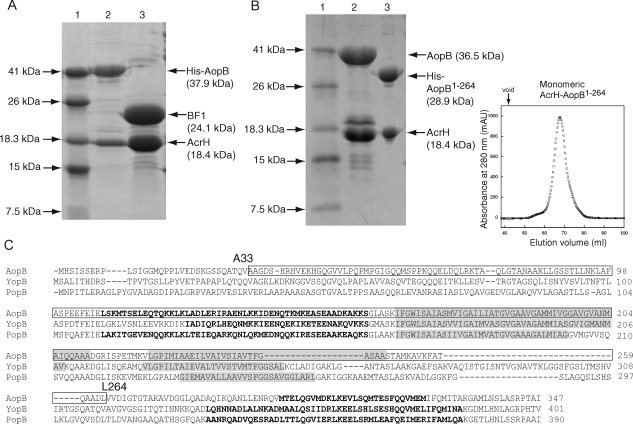
Limited protease digestion of the AcrH-AopB complex. A: SDS-PAGE showing partial digestion of AcrH-AopB with elastase. Lane (1) Mw marker; lane (2) monomeric AcrH-AopB from gel filtration before digestion; and lane (3) AopB is being digested into a single fragment, BF1 (residues 33–264), whereas AcrH remains intact. B: Coexpression and purification of AcrH-AopB1–264 complex. Lane (1) Mw marker; lane (2) AcrH-AopB complex; lane (3) AcrH-AopB1–264 complex. The gel filtration elution profile shows that the AcrH-AopB1–264 complex exists entirely as a monomeric form. The down arrow above the elution profile indicates the void volume of the column. C: Sequence alignment of AopB from Aeromonoas hydrophila (AAR26341) with YopB from Yersinia enterolitica (AAK69211) and PopB from Pseudomonas aeruginosa (AAG05097). Predicted transmembrane domains (TMHMM Server v. 2.0, http://www.cbs.dtu.dk/services/TMHMM-2.0/) are highlighted in gray. Residues that are determined to be involved in chaperone binding are boxed. Residues from the predicted coiled coil regions by the program COILS33 are boldfaced.
