Abstract
In adult patients with congenital adrenal hyperplasia (CAH), the presence of testicular adrenal rest tumours (TART) is an important complication leading to gonadal dysfunction and infertility. These tumours can be already found in childhood and puberty. In this paper, we review the embryological, histological, biochemical, and clinical features of TART and discuss treatment options.
1. Introduction
Congenital adrenal hyperplasia is an inherited disorder affecting the steroid synthesis of the adrenal gland. In more than 90% of the cases, CAH is caused by CYP21 (21-hydroxylase) deficiency [1, 2]. CYP21 deficiency results in an impaired production of cortisol and mostly of aldosterone. Consequently, pituitary ACTH production is increased leading to hyperplasia of the adrenal glands and overproduction of adrenal androgens with prenatal virilisation of the female external genitalia. The impaired production of cortisol and aldosterone may lead to an Addisonian crisis with salt wasting and dehydration in early life [1].
Nowadays, the diagnosis of the most severe types of CAH can be made at an early age by neonatal screening programs or by prenatal diagnosis in the case of an affected proband, thereby preventing life threatening events [3]. However, in adult CAH patients several complications can develop and in recent years it became clear that some of them might be detected already in childhood. One of the most important and frequently detected complications in male CAH patients is the development of testicular tumours. These tumours were first reported in 1940 by Wilkins et al. [4]. Since then testicular tumours have been described in several papers, mainly case reports [5–9]. Because of the morphological and functional resemblance with adrenal tissue they are called “testicular adrenal rest tumours” (TART).
The reported prevalence of TART varies between 0 and 94% and depends on the selection of the patients (age, hormonal control) and the method of tumour detection [10–16]. Usually only tumours of more than 2 cm are detectable by palpation because of their location within the rete testis. Therefore, the tumours can be easily missed when additional imaging techniques such as ultrasound or magnetic resonance imaging (MRI) are not performed. Screening of TART is not routinely performed in male CAH patients.
2. Morphological Characteristics of TART
TART are benign tumours and in most patients the tumours are present bilaterally. The typical location is within the rete testis. Histologically, TART resemble adrenocortical tissue [17–19]. The tumours are not encapsulated and consist of sheets or confluent cords of large polygonal cells with abundant eosinophilic cytoplasm (Figure 1). However, a clear histological differentiation between TART and malignant Leydig cell tumours may be difficult. Some clinical features can help to distinguish between these tumours: TART are bilateral in more than 80%, whereas Leydig cell tumours are bilateral in only 3% of the cases. Furthermore, Reinke crystals, which can be found in 25–40% of Leydig cell tumours, are absent in TART. Malignant degeneration is seen in 10% of Leydig cell tumours but has never been described in patients with TART.
Figure 1.
Testicular adrenal rest tumour growing into rete testis (RT) (HE, original magnification x 200).
3. The Aetiology and Functional Features of TART
Until now the aetiology and functional features of the tumours are not completely understood. The clinical observation that high doses of glucocorticoids can reduce tumour size, most probably due to suppression of ACTH secretion, and that tumour growth may be promoted in conditions where ACTH concentration is high, such as in poorly controlled CAH patients or in patients with Nelson's syndrome, suggests the presence of ACTH receptors on tumour cells [7, 8]. Therefore, it is hypothesized that TART arise from aberrant adrenal cells descended in the embryological period together with the testes.
A limited number of in vivo and in vitro studies showed the presence of adrenal specific 11 β-hydroxylated steroids such as 21-deoxycorticosterone (21DB) and 21-deoxycortisol (21DF) in blood taken from the gonadal veins [20–23]. This indicates the presence of adrenal-like tissue in the testes of these CAH patients with 21-hydroxylase deficiency because these steroids can only be synthesized by adrenal specific 11-hydroxylation, without the need of the deficient 21-hydroxylation step. Recently, we described the presence of adrenal specific enzymes CYP11B1 and CYP11B2 as well as of ACTH and angiotensin II (AII) receptors in 16 testicular tumours of eight patients as measured by mRNA expression with quantitative PCR [24]. Therefore, it can be speculated that tumour growth in CAH patients may not only be stimulated by elevated ACTH concentrations but also by elevated AII levels, as present in salt-wasting CAH patients with poor hormonal control.
Interestingly, intensifying of glucocorticoid treatment with suppression of ACTH secretion is not always successful in reducing tumour size and even in well-controlled CAH patients, with normal or suppressed plasma ACTH levels, testicular adrenal rest tumours are found [6, 25, 26]. So most probably other unknown factors have to contribute to tumour growth. In the literature, the presence of ectopic adrenal rest cells in the testes or along the spermatic cord of healthy neonates is reported with a prevalence of up to 15%, mostly described in patients who underwent groin surgical explorations [27, 28]. However, this prevalence is probably underestimated because single adrenal-like cells or small cell groups are very difficult to detect. The presence of these aberrant adrenal cells within the testis is the most probable prerequisite for the development of TART explaining the often observed discrepancy between the development of TART and hormonal control. It is likely that CAH patients without preexisting ectopic adrenal rest cells within their testes will never develop TART.
Our observation that TART can already be detected in early childhood even in adequately treated patients suggests that when adrenal rest cells are present within the testis, even mildly or intermittently increased ACTH (and AII) concentrations may induce proliferation of these cells within the testis. Poor hormonal control with high ACTH levels may accelerate this process. Furthermore, adrenal cells may already be stimulated in utero where there are elevated levels of ACTH.
So, both the concentrations of and the duration of exposure to growth promoting factors are probably important in the pathogenesis of tumour growth. Furthermore, it can be hypothesized that the pubertal rise of LH may give an additional stimulation of tumour growth as LH receptors are found in TART [29], which may explain the increased prevalence of TART in pubertal and postpubertal CAH patients even when there is good hormonal control. Detailed studies focusing on the effect of ACTH, AII, and LH in young, male CAH patients are needed to determine the role of these factors in the development of TART.
In a recent paper, Val et al. suggest that adrenal rest cells may develop from a different population of adrenal like cells. They describe the presence of cells with mixed adrenal and Leydig cell properties within the mouse testis [30]. These cells express adrenal markers such as CYP11B1 and CYP21 and respond to ACTH and HCG incubations. Therefore, TART may also develop from a different population of cells with adrenal features. Of course these findings have to be translated with caution to the human population as mouse fetal testes also express ACTH receptors in contrast to the developing human testes [30].
4. Long-Term Consequences of TART
TART have no malignant features and, therefore, there seems to be no need to remove them at an early stage. However, because of the central localization of the tumours near the mediastinum testis, compression of the seminiferous tubules finally may lead to obstructive azoospermia and irreversible damage of the surrounding testicular tissue. In a recent study, we showed a decreased tubular diameter and a varying degree of peritubular fibrosis and tubular hyalinization in the testicular biopsies of 7 male CAH patients with longstanding bilateral TART and clinical infertility (Figure 2) [17]. Furthermore, we found a severe decrease in the number of germ cells in all patients. In the literature, obstructive azoospermia is described mainly as a result of extratesticular obstruction due to infections or surgical interventions mostly located at the epididymis or vas deferens [31–34]. In these cases, adverse effects of the obstruction on the germinal epithelium or Leydig cells were not reported [32, 34]. This can be explained by the ability of the epididymis to become enlarged to accommodate the sperm cells and to phagocytise and resorb spermatozoa [32]. It can be speculated that in the case of large TART located in the mediastinum testis proximal to the epididymis, the efferent flow in the seminiferous tubules is chronically obstructed without having the possibility of compensatory dilatation of the epididymis. Longstanding obstruction of the seminiferous tubules could then lead to hypospermatogenesis and peritubular fibrosis.
Figure 2.
Testicular biopsy of a patient showing seminiferous tubules with hypospermatogenesis and prominent peritubular fibrosis with increased number of peritubular fibroblasts (arrows), as well as tubular hyalinisation (arrow-head; original magnification x 200).
In addition to the mechanical effects, the tumours may also have a paracrine effect on the surrounding tissue. Steroids produced by the tumour cells may be toxic to the Leydig cells and/or germ cells [35].
The irreversible end stage of longstanding TART is tubular hyalinization with obstruction of the lumen and complete loss of germ cells and Sertoli cells. In contrast to ischemic hyalinization, where a reduced number of Leydig cells are expected, the interstitium of our patients contained a normal or only slightly reduced number of Leydig cells [17]. Therefore, TART represent a very specific cause of obstructive azoospermia, commonly not mentioned in the literature and with more severe clinical consequences than other forms.
5. TART in Childhood
The presence of TART in children is described mostly in case reports [36–38] and only a limited number of studies describe its prevalence in larger populations of children and adults [5, 15, 39, 40]. Avila et al. detected TART by ultrasound in 8 of 38 male CAH patients (age 6–31 years) [15]. The mean age of the patients was 14.8 years and 7 of the 8 patients with TART were below 18 years old. The youngest patient was 6.2 years old. The total number of investigated patients below 18 years of age was not reported. Vanzulli et al. described a prevalence of 27% of TART in a group of 30 CAH patients between 9 and 32 years old [39]. In the 24-investigated patients below 18 years 7 (29%) had TART. However, these studies did not focus on childhood age and did not present information on gonadal function.
Shanklin et al. studied autopsy material of patients with CAH and detected TART in 3 of 7 patients less than 8 weeks old [5]. In our own patient population, we found that in 34 male CAH children (age 2–18 years old) TART are already present in childhood with a prevalence of 24% [40]. The prevalence increased with age. None of the tumours were detectable by palpation and none of the children with testicular tumours showed signs of gonadal dysfunction. In another study of 19 male CAH patients, age 2–10 years old, a similar prevalence of 21% was found [41]. The investigators found significantly lower inhibin B values in the CAH group compared with a healthy control group suggesting that gonadal dysfunction is already present in prepubertal CAH children [41].
6. Proposed Classification of TART
Based on the histological appearance of TART and the surrounding testicular parenchyma and the clinical observations described above, we propose that the development and growth of TART can be divided in five different stages (Table 1 and Figure 3).
Table 1.
Proposed classification of testicular adrenal rests.
| Histological description | Reversibility | Treatment options | |
|---|---|---|---|
| Stage 1 | Presence of adrenal rests within the rete testis—not detectable | +++ | — |
| Stage 2 | Hypertrophy and hyperplasia of adrenal rest cells due to growth stimulating factors (e.g., ACTH, AII) | +++ | Optimizing glucocorticoids |
| Stage 3 | Further growth of the adrenal rest cells with (reversible) compression of the rete testis | ++ | Optimizing glucocorticoids Surgery? |
| Stage 4 | Induction of fibrosis and focal lymphocytic infiltrates | −/+ | Surgery? |
| Stage 5 | Irreversible damage of testicular parenchyma. | − | — |
| Parts of the tumour are replaced by adipose tissue |
Figure 3.
Schematic view of the proposed classification of testicular adrenal rests.
Stage 1 —
This stage can be defined as the presence of adrenal rest cells within the rete testis, not detectable by scrotal ultrasound. In healthy boys, these cells probably regress in utero or in the first years of life.
Stage 2 —
In CAH patients, the adrenal rest cells may proliferate in the presence of increased concentrations of growth promoting factors such as ACTH (and possibly also of AII). In this stage, the adrenal rest cells may become visible by ultrasound as one or more small hypoechogenic lesions. The age of onset of cell growth may depend on the cumulative exposure to ACTH (and AII) concentrations over time and the number of ACTH (and AII) receptors on the adrenal rest cells.
Stage 3 —
Further growth of the adrenal rest cells will compress the rete testis. In pubertal or postpubertal CAH patients, oligo- or azoospermia may already be found due to obstruction of the seminiferous tubules. Signs of gonadal dysfunction such as decreased inhibin B and increased FSH and LH levels may also be present. At this stage, tumour size may still be reduced by high dosages of glucocorticoids. However, because it can be expected that tumour growth will restart after decreasing the dose of glucocorticooids, this is only a temporary solution.
Stage 4 —
Further hypertrophy and hyperplasia of the adrenal rest cells with progressive obstruction of the rete testis may lead to induction of fibrosis within the tumour and focal lymphocytic infiltration. Several small tumours within the rete testis will confluate, forming a single-lobulated structure separated from the residual testicular tissue by fibrous strands. In this stage, high doses of glucocorticoids are probably no longer effective in decreasing tumour size because parts of the tumours consist of fibrous tissue and/or because the adrenal rest cells may dedifferentiate in time with loss of ACTH and AII dependency. Furthermore, peritubular fibrosis can be found in the surrounding testicular tissue indicating early testicular damage.
Stage 5 —
Chronic obstruction subsequently will lead to destruction of the surrounding testicular parenchyma with irreversible damage of the testis.
Further studies are necessary to validate this proposed classification of TART.
7. Diagnosis of TART (Table 2)
Table 2.
Diagnosis and follow up of TART in male patients with congenital adrenal hyperplasia.
| (i) Scrotal ultrasound 1 x/year from 8 years onwards |
|---|
| (ii) Biochemical analysis 1 x/year: |
| (1) Hormonal control: ACTH, renin, |
| 17-hydroxyprogesterone, androstenedione |
| (2) Gonadal function: LH, FSH, inhibin B |
| (iii) Semen analysis in pubertal and adult patients |
| (iv) Cryopreservation of semen |
| (v) When considering surgery: testicular biopsy |
Because the location of the tumours within the rete testis TART are difficult to palpate, usually only tumours with a size of more than 2 cm are detectable by palpation. Ultrasound and MRI are equally good methods for detection and monitoring of the tumours (Figures 4 and 5), but ultrasound is preferable because it is quick and cheap [26, 42] and even very small adrenal rests of only a few millimetres in diameter are detectable. However, until now very small adrenal rests or single cells (stage 1) cannot be detected even not with radiological techniques. In stage 2, testicular tumours can be visible as small hypoechogenic lesions. From stage 3 onwards fibrous strands can be visible as hyperechogenic reflections. In our clinic, we start routinely yearly scrotal ultrasound screening from 8 years onwards.
Figure 4.
Scrotal ultrasound of a 13-year-old male CAH patient. Transverse image shows a mostly hypoechogenic rounded lesion in the left testis near the rete testis.
Figure 5.
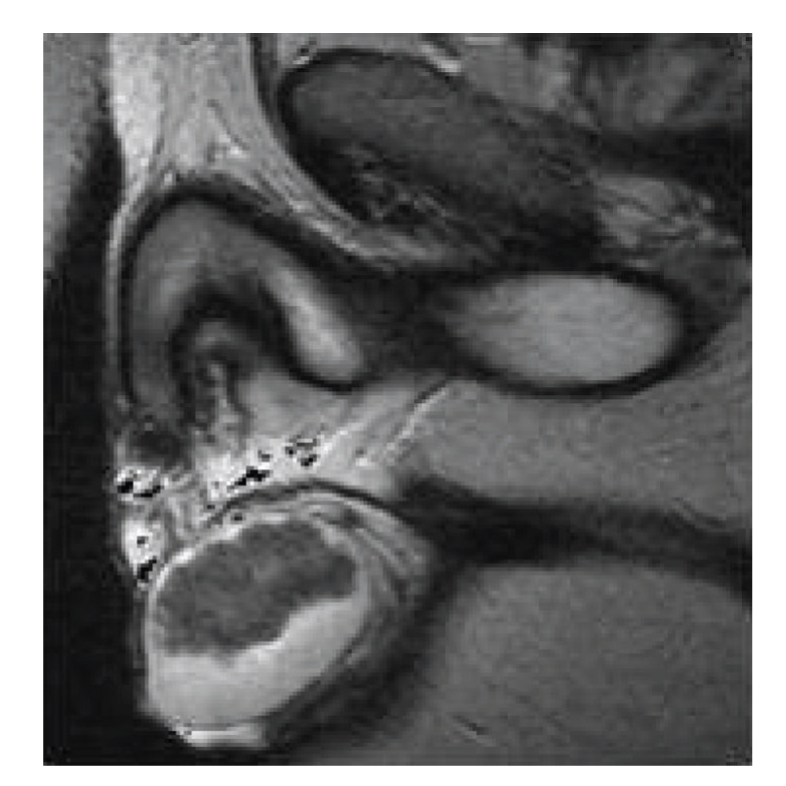
T2-weighted MR image of longstanding bilateral testicular adrenal rest tumours in a 33-year-old patient. Note that heterogeneous low-signal-intensity tumours are displacing surrounding high signal normal testicular tissue.
Evaluation of gonadal function by determining blood LH, FSH, inhibin B, and testosterone concentrations can help to determine the degree of gonadal failure as is expected from stage 3 onwards. It should be realized that LH and FSH are of limited value to evaluate gonadal function in CAH patients because the gonadotropines may be suppressed due to elevated adrenal androgens, which are partly aromatized to estrone and estradiol [43]. In contrast to other causes of hypogonadotropic, hypogonadism CAH patients have generally normal or only slightly decreased testosterone levels because of elevated adrenal androgens. Inhibin B is a better marker for the evaluation of Sertoli cell function and may also be used in the evaluation of gonadal function in prepubertal children [44]. Semen analysis can be performed in (post) pubertal and adult patients when the patient is willing to collect semen for analysis.
Because tumour growth may be related to hormonal control as discussed earlier, it is important to monitor serum ACTH, renin, 17-hydroxyprogesterone, and androstenedione concentrations.
In the case of longstanding tumours in infertile CAH patients, a testicular biopsy may be helpful to evaluate the quality of residual testicular parenchyma (stage 4 or 5) [17]. Such a biopsy is strongly advised before surgical treatment is offered. However, one should realize that a testicular biopsy only gives information about a limited area of the testis.
Because only patients in whom aberrant adrenal cells have been nestled in the testes in the embryological period are supposed to be at risk for developing TART, it would be very important to identify these patients as early as possible. Nowadays, adrenal rests can only be detected after substantial growth. In our own centre, we start screening from the age of 8 years old. However, age of screening in childhood is still controversial because of the limited experience with treatment options in childhood. In the future, new sensitive imaging techniques may help to detect these adrenal rests in the first years of life. If this is possible, patients with adrenal rests within their rete testis could be monitored and treated more intensively, whereas in patients without adrenal rests unnecessary ultrasound follow up and aggressive treatment strategies possibly could be avoided.
8. Treatment of TART
Until now, intensifying glucocorticoid treatment is the choice of treatment in patients with TART. Intensifying glucocorticoid therapy may lead to reduction of the tumour size by suppression of ACTH secretion thereby improving testicular function in stages 2 and 3. However, though case reports with successful pregnancy of partners of male CAH patients have been published [45], some studies report failure of intensified glucocorticoid treatment and serious side effects after long-standing dexamethasone treatment and, therefore, some of the patients will not accept this treatment option [26, 45, 46]. Furthermore, it may be that this treatment leads only to temporary improvement of the obstruction because tumour growth may start again after lowering the glucocorticoid dose. However, optimizing glucocorticoid medication especially in patients with poor hormonal control is important to determine whether tumour growth is reversible (stage 3). Because also AII may stimulate tumour growth also the mineralocorticoid suppletion has to be optimized [24].
In stage 4, increasing the dose of glucocorticoids is probably no longer effective in decreasing tumour size, but removal of the tumour may prevent further testicular damage. Because of the benign character of the tumours, testis-sparing surgery has been proposed for the treatment of TART. Walker et al. performed testis-sparing surgery in 3 adult CAH patients [25]. Postoperatively, there was good vascular flow and no recurrence of the tumour. Tiryaki et al. reported 2 CAH patients with steroid unresponsive testicular tumours, who were also treated by testis-sparing surgery [47]. In both studies, no information about pituitary-gonadal function before and after surgery was reported. In a recent study, we showed that in patients with longstanding TART (stage 5) gonadal dysfunction did not improve suggesting irreversible damage of the surrounding testicular tissue [44]. Furthermore, additional damage from surgery could not be excluded. From our experience, we now conclude that in this stage the only indication for surgery is the relief of pain and discomfort caused by TART. Therefore, mainly in longstanding TART with signs of gonadal dysfunction, testicular biopsies are advised to evaluate the quality of the surrounding testicular parenchyma, before surgery is considered.
Testicular surgery in CAH children with TART has not yet been performed. Further studies in childhood are needed to investigate whether surgery in stages 2, 3, and 4 may prevent irreversible damage to the testes. As long as medical and surgical treatments of TART are far from perfect, patients should be informed about the negative effects of TART on fertility and cryopreservation of semen should be offered as soon as possible. Because adrenal rest cells are already present in the embryological period, it is clear that prevention of TART is not possible.
9. Conclusion
TART is the most important cause of infertility in adult male CAH patients. They are not malignant but longstanding TART can result in irreversible damage of testicular tissue and subsequently infertility. TART is also detectable in children with CAH but the presence of gonadal dysfunction due to TART in childhood is controversial. TART have histological and functional features of adrenocortical tissue and growth can be stimulated by elevated ACTH concentrations. Intensifying glucocorticoid therapy is the first step in the treatment of TART. Before testis-sparing surgery is considered, testicular biopsies are advised to evaluate the quality of the surrounding testicular parenchyma.
References
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. The New England Journal of Medicine. 2003;349(8):776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.Forest MG. Recent advances in the diagnosis and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Human Reproduction Update. 2004;10(6):469–485. doi: 10.1093/humupd/dmh047. [DOI] [PubMed] [Google Scholar]
- 3.Grosse SD, Van Vliet G. How many deaths can be prevented by newborn screening for congenital adrenal hyperplasia? Hormone Research. 2007;67(6):284–291. doi: 10.1159/000098400. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins L, Fleishmann W, Howard JE. Macrogenitosomia precox associated with hyperplasia of the androgenic tissue of the adrenal and death from corticoadrenal insufficiency. Endocrinology. 1940;26:385–395. [Google Scholar]
- 5.Shanklin DR, Richardson AP, Jr., Richardson G. Testicular hilar nodules in adrenogenital syndrome. The nature of the nodules. American Journal of Diseases of Children. 1963;106:243–250. doi: 10.1001/archpedi.1963.02080050245001. [DOI] [PubMed] [Google Scholar]
- 6.Rich MA, Keating MA, Levin HS, Robert K. Tumors of the adrenogenital syndrome: an aggressive conservative approach. The Journal of Urology. 1998;160(5):1838–1841. [PubMed] [Google Scholar]
- 7.Bonaccorsi AC, Adler I, Figueiredo JG. Male infertility due to congenital adrenal hyperplasia: testicular biopsy findings, hormonal evaluation, and therapeutic results in three patients. Fertility and Sterility. 1987;47(4):664–670. doi: 10.1016/s0015-0282(16)59119-5. [DOI] [PubMed] [Google Scholar]
- 8.Giacaglia LR, Mendonca BB, Madureira G, et al. Adrenal nodules in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency: regression after adequate hormonal control. Journal of Pediatric Endocrinology & Metabolism. 2001;14(4):415–419. doi: 10.1515/jpem.2001.14.4.415. [DOI] [PubMed] [Google Scholar]
- 9.Rutgers JL, Young RH, Scully RE. The testicular “tumor” of the adrenogenital syndrome. A report of six cases and review of the literature on testicular masses in patients with adrenocortical disorders. American Journal of Surgical Pathology. 1988;12(7):503–513. [PubMed] [Google Scholar]
- 10.Jääskeläinen J, Kiekara O, Hippeläinen M, Voutilainen R. Pituitary gonadal axis and child rate in males with classical 21-hydroxylase deficiency. Journal of Endocrinological Investigation. 2000;23(1):23–27. doi: 10.1007/BF03343671. [DOI] [PubMed] [Google Scholar]
- 11.Cabrera MS, Vogiatzi MG, New MI. Long term outcome in adult males with classic congenital adrenal hyperplasia. The Journal of Clinical Endocrinology & Metabolism. 2001;86(7):3070–3078. doi: 10.1210/jcem.86.7.7668. [DOI] [PubMed] [Google Scholar]
- 12.Stikkelbroeck NMML, Otten BJ, Pasic A, et al. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. The Journal of Clinical Endocrinology & Metabolism. 2001;86(12):5721–5728. doi: 10.1210/jcem.86.12.8090. [DOI] [PubMed] [Google Scholar]
- 13.Otten BJ, Stikkelbroeck MML, Claahsen-van der Grinten HL, Hermus ARMM. Puberty and fertility in congenital adrenal hyperplasia. Endocrine Development. 2005;8:54–66. doi: 10.1159/000084093. [DOI] [PubMed] [Google Scholar]
- 14.Urban MD, Lee PA, Migeon CJ. Adult height and fertility in men with congenital virilizing adrenal hyperplasia. The New England Journal of Medicine. 1978;299(25):1392–1396. doi: 10.1056/NEJM197812212992505. [DOI] [PubMed] [Google Scholar]
- 15.Avila NA, Premkumar A, Shawker TH, Jones JV, Laue L, Cutler GB., Jr. Testicular adrenal rest tissue in congenital adrenal hyperplasia: findings at gray-scale and color Doppler US. Radiology. 1996;198(1):99–104. doi: 10.1148/radiology.198.1.8539414. [DOI] [PubMed] [Google Scholar]
- 16.Avila NA, Premkumar A, Merke DP. Testicular adrenal rest tissue in congenital adrenal hyperplasia: comparison of MR imaging and sonographic findings. American Journal of Roentgenology. 1999;172(4):1003–1006. doi: 10.2214/ajr.172.4.10587136. [DOI] [PubMed] [Google Scholar]
- 17.Claahsen-van der Grinten HL, Otten BJ, Hermus ARMM, Sweep FCGJ, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertility and Sterility. 2008;89(3):597–601. doi: 10.1016/j.fertnstert.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Rich MA, Keating MA. Leydig cell tumors and tumors associated with congenital adrenal hyperplasia. Urologic Clinics of North America. 2000;27(3):519–528. doi: 10.1016/s0094-0143(05)70099-9. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen JL, Savage A, Mobb GE. The testicular ‘tumour’ of adrenogenital syndrome—a persistent diagnostic pitfall. Histopathology. 1991;19(5):468–470. doi: 10.1111/j.1365-2559.1991.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 20.Clark RV, Albertson BD, Munabi A, et al. Steroidogenic enzyme activities, morphology, and receptor studies of a testicular adrenal rest in a patient with congenital adrenal hyperplasia. The Journal of Clinical Endocrinology & Metabolism. 1990;70(5):1408–1413. doi: 10.1210/jcem-70-5-1408. [DOI] [PubMed] [Google Scholar]
- 21.Bercovici JP, Fiet J, Gibault L, et al. Testicular adrenal rest tumours in salt wasting congenital adrenal hyperplasia (in vivo and in vitro studies) Journal of Steroid Biochemistry and Molecular Biology. 2005;93(1):67–72. doi: 10.1016/j.jsbmb.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Combes-Moukhovsky ME, Kottler ML, Valensi P, Boudou P, Sibony M, Attali JR. Gonadal and adrenal catheterization during adrenal suppression and gonadal stimulation in a patient with bilateral testicular tumors and congenital adrenal hyperplasia. The Journal of Clinical Endocrinology & Metabolism. 1994;79(5):1390–1394. doi: 10.1210/jcem.79.5.7962333. [DOI] [PubMed] [Google Scholar]
- 23.Blumberg-Tick J, Boudou P, Nahoul K, Schaison G. Testicular tumors in congenital adrenal hyperplasia: steroid measurements from adrenal and spermatic veins. The Journal of Clinical Endocrinology & Metabolism. 1991;73(5):1129–1133. doi: 10.1210/jcem-73-5-1129. [DOI] [PubMed] [Google Scholar]
- 24.Claahsen-van der Grinten HL, Otten BJ, Sweep FCGJ, et al. Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. The Journal of Clinical Endocrinology & Metabolism. 2007;92(9):3674–3680. doi: 10.1210/jc.2007-0337. [DOI] [PubMed] [Google Scholar]
- 25.Walker BR, Skoog SJ, Winslow BH, Canning DA, Tank ES. Testis sparing surgery for steroid unresponsive testicular tumors of the adrenogenital syndrome. The Journal of Urology. 1997;157(4):1460–1463. [PubMed] [Google Scholar]
- 26.Stikkelbroeck NMML, Hermus ARMM, Suliman HM, Jager GJ, Otten BJ. Asymptomatic testicular adrenal rest tumours in adolescent and adult males with congenital adrenal hyperplasia: basal and follow-up investigation after 2.6 years. Journal of Pediatric Endocrinology & Metabolism. 2004;17(4):645–653. doi: 10.1515/jpem.2004.17.4.645. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan JG, Gomel M, Kinder RB. Ectopic adrenocortical tissue found at groin exploration in children: incidence in relation to diagnosis, age and sex. BJU International. 2005;95(3):407–410. doi: 10.1111/j.1464-410X.2005.05310.x. [DOI] [PubMed] [Google Scholar]
- 28.Souverijns G, Peene P, Keuleers H, Vanbockrijck M. Ectopic localisation of adrenal cortex. European Radiology. 2000;10(7):1165–1168. doi: 10.1007/s003309900263. [DOI] [PubMed] [Google Scholar]
- 29.Benvenga S, Smedile G, Lo Giudice FL, Trimarchi F. Testicular adrenal rests: evidence for luteinizing hormone receptors and for distinct types of testicular nodules differing for their autonomization. European Journal of Endocrinology. 1999;141(3):231–237. doi: 10.1530/eje.0.1410231. [DOI] [PubMed] [Google Scholar]
- 30.Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Developmental Biology. 2006;299(1):250–256. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Behre HM, Nieschlag E, Meschede D. Obstructions of the seminal ducts. In: Nieschlag E, Behre H, editors. Andrology. 2nd edition. New York, NY, USA: Springer; 2001. pp. 179–181. [Google Scholar]
- 32.Wong TW, Straus FH, II, Warner NE. Testicular biopsy in the study of male infertility—II. Posttesticular causes of infertility. Archives of Pathology. 1973;95(3):160–164. [PubMed] [Google Scholar]
- 33.Dondero F, Lombardo F. Male infertility. In: Wass JA, Shalet SM, editors. Oxford Textbook of Endocrinology and Diabetes. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 34.Belmonte IG, de Serrano MNM. Partial obstruction of the seminal path, a frequent cause of oligozoospermia in men. Human Reproduction. 1998;13(12):3402–3405. doi: 10.1093/humrep/13.12.3402. [DOI] [PubMed] [Google Scholar]
- 35.Murphy H, George C, de Kretser D, Judd S. Successful treatment with ICSI of infertility caused by azoospermia associated with adrenal rests in the testes. Human Reproduction. 2001;16(2):263–267. doi: 10.1093/humrep/16.2.263. [DOI] [PubMed] [Google Scholar]
- 36.Boulware SD. Case report. An unusual case of precocious puberty. Current Opinion in Pediatrics. 1997;9(4):443–446. doi: 10.1097/00008480-199708000-00022. [DOI] [PubMed] [Google Scholar]
- 37.Erdogan S, Ergin M, Cevlik F, et al. Testicular adrenal rest hyperplasia due to 21-hydroxylase deficiency: a case report. Endocrine Pathology. 2006;17(1):83–87. doi: 10.1385/ep:17:1:83. [DOI] [PubMed] [Google Scholar]
- 38.Willi U, Atares M, Prader A, Zachmann M. Testicular adrenal-like tissue (TALT) in congenital adrenal hyperplasia: detection by ultrasonography. Pediatric Radiology. 1991;21(4):284–287. doi: 10.1007/BF02018626. [DOI] [PubMed] [Google Scholar]
- 39.Vanzulli A, DelMaschio A, Paesano P, et al. Testicular masses in association with Adrenogenital syndrome: US findings. Radiology. 1992;183(2):425–429. doi: 10.1148/radiology.183.2.1561344. [DOI] [PubMed] [Google Scholar]
- 40.Claahsen-van der Grinten HL, Sweep FCGJ, Blickman JG, Hermus ARMM, Otten BJ. Prevalence of testicular adrenal rest tumors in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. European Journal of Endocrinology. 2007;157(3):339–344. doi: 10.1530/EJE-07-0201. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Aguayo A, Rocha A, Rojas N, et al. Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia. The Journal of Clinical Endocrinology & Metabolism. 2007;92(12):4583–4589. doi: 10.1210/jc.2007-0383. [DOI] [PubMed] [Google Scholar]
- 42.Stikkelbroeck NMML, Suliman HM, Otten BJ, Hermus ARMM, Blickman JG, Jager GJ. Testicular adrenal rest tumours in postpubertal males with congenital adrenal hyperplasia: sonographic and MR features. European Radiology. 2003;13(7):1597–1603. doi: 10.1007/s00330-002-1786-3. [DOI] [PubMed] [Google Scholar]
- 43.Claahsen-van der Grinten HL, Stikkelbroeck NMML, Sweep CGJ, Hermus ARMM, Otten BJ. Fertility in patients with congenital adrenal hyperplasia. Journal of Pediatric Endocrinology & Metabolism. 2006;19(5):677–685. doi: 10.1515/jpem.2006.19.5.677. [DOI] [PubMed] [Google Scholar]
- 44.Claahsen-van der Grinten HL, Otten BJ, Takahashi S, et al. Testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia: evaluation of pituitary-gonadal function before and after successful testis-sparing surgery in eight patients. The Journal of Clinical Endocrinology & Metabolism. 2007;92(2):612–615. doi: 10.1210/jc.2006-1311. [DOI] [PubMed] [Google Scholar]
- 45.Claahsen-van der Grinten HL, Otten BJ, Sweep FCGJ, Hermus ARMM. Repeated successful induction of fertility after replacing hydrocortisone with dexamethasone in a patient with congenital adrenal hyperplasia and testicular adrenal rest tumors. Fertility and Sterility. 2007;88(3):705.e5–705.e8. doi: 10.1016/j.fertnstert.2006.11.148. [DOI] [PubMed] [Google Scholar]
- 46.Cutfield RG, Bateman JM, Odell WD. Infertility caused by bilateral testicular masses secondary to congenital adrenal hyperplasia (21-hydroxylase deficiency) Fertility and Sterility. 1983;40(6):809–814. doi: 10.1016/s0015-0282(16)47485-6. [DOI] [PubMed] [Google Scholar]
- 47.Tiryaki T, Aycan Z, Hücümenoğlu S, Atayurt H. Testis sparing surgery for steroid unresponsive testicular tumors of the congenital adrenal hyperplasia. Pediatric Surgery International. 2005;21(10):853–855. doi: 10.1007/s00383-005-1547-x. [DOI] [PubMed] [Google Scholar]