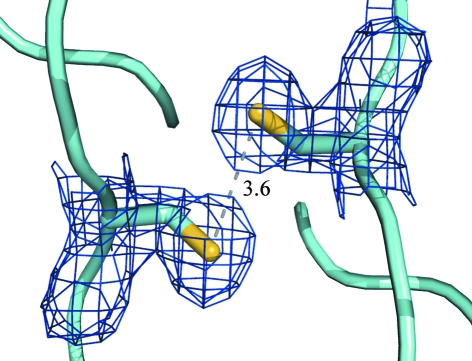
Figure 5.
Two cysteine residues shown using 2F o − F c electron density contoured at 1σ (0.28 e Å−3) reside at the homodimer interface and are reduced with no disulfide formation. However, the distance (shown in Å) between these indicates the possibility of disulfide formation under oxidative conditions, which may confer additional protein stability.