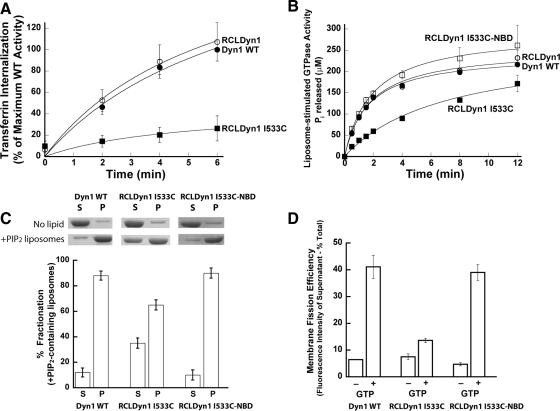
Figure 1.
I533C mutation in VL1 impairs dynamin function in vivo and in vitro. (A) Transferrin uptake was followed in tTA-HeLa cells infected for 16–18 h with recombinant adenovirus encoding Dyn1 WT (●), RCLDyn1 (○), or RCLDyn1 I533C (■). The kinetics of internalization is plotted as a percentage of maximum uptake by Dyn1 WT. (B) Lipid-stimulated GTPase activities for 0.5 μM Dyn1 WT (●), RCLDyn1 (○), RCLDyn1 I533C (■), or RCLDyn1 I533C-NBD (□) preincubated with PIP2-containing liposomes (150 μM total lipid) were measured at 37°C. The concentration of Pi released is plotted as a function of time. (C) Binding of 1.0 μM Dyn1 WT, RCLDyn1 I533C, or RCLDyn1 I533C-NBD to PIP2-containing liposomes (300 μM lipid) was examined by sedimentation followed by SDS-PAGE analyses of supernatant (S) and pellet (P) fractions. Densitometric analyses of S and P fractions obtained using an Alpha Innotec Imager and FluorChem SP software (San Leandro, CA) are plotted. (D) Membrane fission activities of 0.5 μM Dyn 1 WT, unlabeled RCLDyn1 I533C, or fluorescently labeled RCLDyn1 I533C-NBD on RhPE-labeled SUPER templates (6 μM total lipid) in the constant presence of GTP (1 mM) were determined by a low-speed sedimentation assay as described in Materials and Methods. The fluorescence intensity of RhPE-labeled vesicles generated by membrane fission and recovered in the supernatant is plotted as a percentage of the total RhPE-labeled templates added to the assay. All values reported in this study represent the mean ± SD of at least three independent experiments.