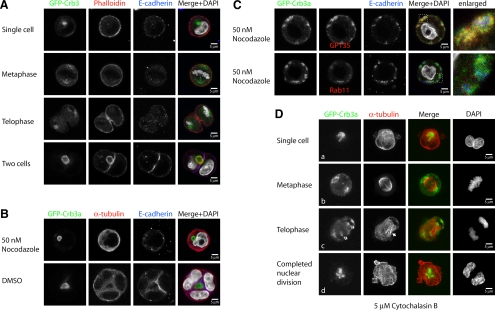
Figure 4.
Internalization and trafficking of GFP-Crb3 is independent of actin polymerization. GFP-Crb3a–expressing MDCKII cells were embedded in Geltrex as described, fixed at different times, and stained with the indicated antibodies or phalloidin. For tubulin inhibition experiments (B and C, single Z-sections), the 2% Geltrex-containing medium was supplemented with 50 nM nocodazole or an equal volume of DMSO. Actin inhibition experiments (D) were performed in the presence of 5 μM cytochalasin B. (A) Control cells, single Z-sections. GFP-Crb3a does not colocalize with actin-positive structures until the nascent lumen is formed. (B) Inhibition of tubulin polymerization. At 36 h after embedding, uninhibited cells (DMSO) have mostly reached the four-cell stage, whereas nocodazole treatment inhibited cell division in all observed cells. (C) Nocodazole-treated cells did not form solitary lumina, but showed small diffusely distributed structures localizing adjacent to the plasma membrane. GFP-Crb3a–positive structures colocalize with GP135, but not E-cadherin or Rab11 (see enlarged parts of merged images). (D, collapsed Z-stacks) Cytochalasin-treated cells. Inhibition of actin polymerization with cytochalasin B does not impair internalization of GFP-Crb3a, its transport to the spindle poles and to the medial side of the two daughter nuclei (outlined arrows). The forming midzone microtubules (solid arrow) fail to get bundled as a result of the inhibited cytokinesis.