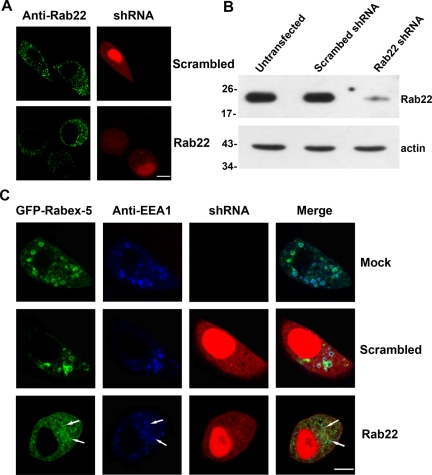
Figure 4.
Blocking Rab22 expression via shRNA abrogates direct membrane targeting and function of Rabex-5. (A) Confocal fluorescence microscopy showing knockdown of endogenous Rab22 by Rab22-specific shRNA in HeLa cells. Cells were transfected with pSIREN-RetroQ-DsRed-Express constructs expressing either a Rab22-specific or a scrambled shRNA as indicated. Transfected cells were identified by dsRed expression whose red fluorescence is distributed throughout cytoplasm and in nucleus. Rab22 in transfected cells (with red fluorescence) as well as untransfected cells (without red fluorescence) was identified by immunofluorescence microscopy with the anti-Rab22 antibody (green). The results were reproducible in two independent experiments. Bar, 8 μm. B. Immunoblot confirming the knockdown of endogenous Rab22 by Rab22-specific shRNA in HeLa cells. Cell lysates from either untransfected cells as a control or transfected cells expressing scrambled or Rab22-specific shRNA were subjected to immunoblot analysis with the anti-Rab22 antibody. The same membrane was also probed with the anti-actin antibody, as a loading control. Molecular mass standards (in kilodaltons) are indicated on the left side of the panels. The results were reproducible in three independent experiments. (C) Confocal fluorescence microscopy showing that shRNA-mediated Rab22 knockdown blocks membrane targeting of Rabex-5 to early endosomes in HeLa cells. Cells were either mock transfected as a control or transfected with the pSIREN-RetroQ-DsRed-Express constructs expressing Rab22-specific or scrambled shRNAs as indicated (red). GFP-Rabex-5 (green) was coexpressed in these cells via pBI vector. At 48 h posttransfection, the cells were fixed, permeabilized, and immunostained with the anti-EEA1 polyclonal antibody (blue), followed by confocal fluorescence microscopy. The results were reproducible in three independent experiments. Bar, 8 μm.