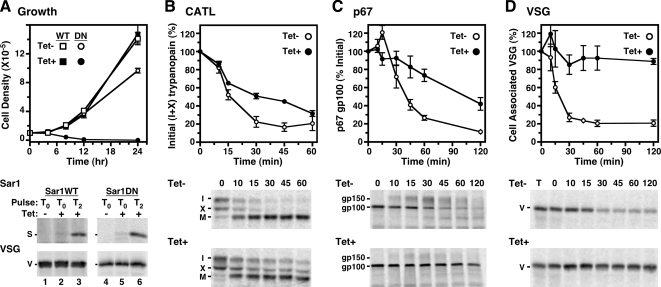
Figure 1.
TbSar1DN expression is lethal and delays forward protein trafficking in T. brucei. (A–D) Transgenic cell lines containing the inducible TbSar1WT (squares, A only) and TbSar1DN (circles, all panels) constructs were cultured without (Tet−, open symbols) or with tetracycline (Tet+, closed symbols) to induce expression of the wild-type and dominant-negative TbSar1 proteins. (A) Growth of the TbSar1WT and TbSar1DN cell lines were monitored (top, mean ± SD for triplicate cultures), and cells were metabolically radiolabeled (bottom) for 1 h in the absence (lanes 1 and 4) or presence of tetracycline after 0 h (lanes 2 and 5) or 2 h (lanes 3 and 6) of prior tetracycline induction. As indicated, lysates were immunoprecipitated with anti-T7 for TbSar1 proteins (S, top images) or anti-VSG (V, bottom images) as a labeling/loading control. Precipitates were analyzed by SDS-PAGE (107 cell equivalents/lane) and phosphorimaging. (B–D) Control (Tet−) and induced (Tet+, 2.5 h) TbSar1DN cells were pulse-chase radiolabeled as described in Materials and Methods, and trafficking of endogenous TbbCATL (B), p67 (C), and VSG (D) was followed by immunoprecipitation and SDS-PAGE (5 × 106 cell equivalents/lane, TbbCATL and p67; 3 × 105 cell equivalents/lane, VSG). Phosphorimages of representative gels are presented for each reporter (bottom). The rates of turnover (top) were quantified (mean ± SEM for triplicate independent experiments) as disappearance of the initial forms for TbbCATL (I + X) and for p67 (gp100) and as loss of cell-associated VSG during hypotonic lysis as a percentage of total VSG in a time zero whole cell lysate (T).