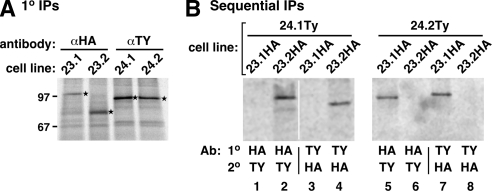
Figure 5.
TbSec23.1/TbSec24.2 and TbSec23.2/TbSec24.1 form obligate physical pairs. TbSec subunits were in situ epitope tagged (TbSec23.1 and TbSec23.2, HA tag; TbSec24.1 and TbSec24.2, Ty tag), in all possible HA/Ty double combinations, by homologous recombination as described in Materials and Methods. (A) Tagged cell lines were pulse radiolabeled (2 h) and lysed in standard RIPA buffer. Labeled polypeptides were specifically immunoprecipitated from cell extracts with anti-epitope antibodies as indicated and analyzed by SDS-PAGE (107 cell equivalents/lane) and phosphorimaging. Bands corresponding to the expected size of each TbSec component (TbSec23.1:HA, 106.6 kDa; TbSec23.2:HA, 83.3 kDa; TbSec24.1:Ty, 108.1 kDa; and TbSec24.2:Ty, 100.8 kDa) are indicated (stars). These bands were not immunoprecipitated from untagged control cell lines (data not shown). (B) Physical interactions were determined by sequential immunoprecipitations using the doubly HA/Ty-tagged cell lines as described in Materials and Methods. Each cell line was radiolabeled (2 h), lysed under nondenaturing conditions, and immunoprecipitated first with either anti-HA or anti-Ty antibodies as indicated (1°). Specific immunoprecipitates were solubilized under denaturing conditions and reprecipitated with the alternate antibody (2°, anti-Ty or anti-HA). Final precipitates were analyzed by SDS-PAGE (107 cell equivalents/lane) and phosphorimaging. The vertical white line between lanes 2 and 3 indicates intervening lanes that were digitally excised for the sake of presentation (all remaining lanes from same exposure).