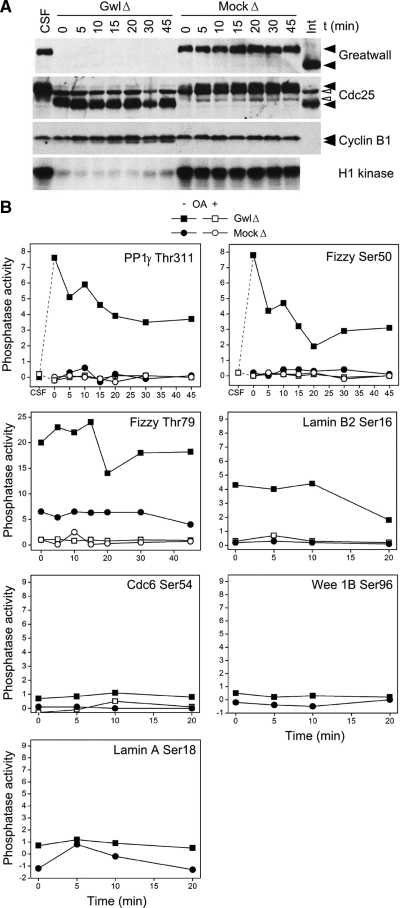
Figure 1.
Gwl depletion from CSF extracts induces OA-sensitive phosphatase(s) directed against CDK phosphosites. (A) Depletion of Gwl from CSF extracts causes pseudomitotic exit. Immunodepletion was performed as described (Yu et al., 2006) by incubating protein A beads coated with affinity-purified anti-Gwl for 1 h at 4°C and then removing the beads by centrifugation. The supernatant was then incubated at 22°C starting at t = 0. Mock-depleted (MockΔ) control extracts were treated with protein A beads alone. MockΔ and CSF extracts remain in M phase as demonstrated by M phase-specific phosphorylations of Gwl and Cdc25, as well as high histone H1 kinase activity (measuring CDK function). In contrast, Gwl-depleted (GwlΔ) extracts have low H1 kinase, and Cdc25 migrates at its interphase (Int) position. During this pseudomitotic exit, cyclin B1 remains undegraded, whereas inhibitory phosphorylations accumulate on Thr14 and Tyr15 of Cdk1 (Yu et al., 2006; see also Figure 2 below). The white arrowheads point to nonspecific background bands. (B) Induction of anti-CDK phosphatase activity after Gwl depletion. Three-microliter aliquots of the extracts shown in A were assayed for phosphatase activity as described (Mochida and Hunt, 2007), with or without the addition of 2.5 μM okadaic acid (OA). The substrates for this assay were MBP fusion proteins labeled in vitro with [γ-32P]ATP by Cdk2/cyclin A; the fusion proteins contained ∼25 residue regions surrounding known CDK targets (for details, see Mochida and Hunt, 2007). The y-axes of the graphs show the percentage of radioactive phosphate released from the input substrate. Dephosphorylations of substrates containing the sites Thr311 of the γ isoform of PP1, Ser50 and Thr79 of Fizzy/Cdc20, and Ser16 of Lamin B2 are strongly induced by Gwl depletion and are OA-sensitive. As previously noted (Mochida and Hunt, 2007), the Fizzy Thr79 phosphosite is dephosphorylated at a low rate during M phase (as seen in mock-depleted extracts), whereas dephosphorylation of the other three substrates is undetectable during M phase. The decay over time in the phosphatase activity of GwlΔ likely reflects the slow reacquisition of the extract's M phase characteristics due to the continued synthesis of Gwl and cyclins from endogenous mRNAs (Yu et al., 2006). The other assayed substrates shown in the figure (Ser54 of Cdc6, Ser96 of Wee1B, and Ser18 of Lamin A) are not obviously targeted by the phosphatase induced by Gwl depletion.