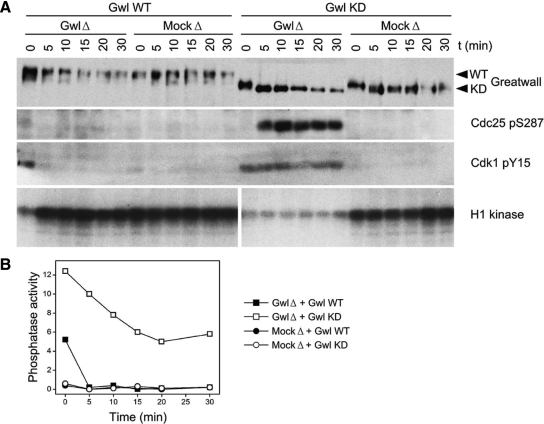
Figure 2.
Rescue of Gwl depletion from CSF extracts with exogenous Gwl protein. (A) Pseudomitotic exit caused by Gwl depletion (GwlΔ) of CSF extracts can be reversed by the addition of active wild-type (WT) but not kinase-dead (KD) Gwl protein, as judged by the interphase-specific phosphorylations of Cdc25 Ser287 (Stanford and Ruderman, 2005) and Cdk1 Tyr15 (Yu et al., 2006; Zhao et al., 2008), and the M phase-specific phosphorylation of histone H1 by CDKs. Mock-depleted (MockΔ) extracts remain in M phase regardless of the nature of the exogenously added protein. The exogenous proteins were purified from OA-treated Sf9 insect cells expressing baculovirus constructs (Zhao et al., 2008), and were added in threefold excess with respect to endogenous Gwl. Gwl proteins were added immediately after depletion at 4°C (t = 0), and the samples were then incubated at 22°C. (B) Phosphatase assays of the same samples shown in A, using the substrate containing the Thr311 PP1γ phosphosite. Phosphatase activity was consistently very low during M phase, but elevated during interphase (that is, in the GwlΔ samples supplemented with Gwl KD). The intermediate level of phosphatase activity observed in the GwlΔ sample supplemented with Gwl WT at t = 0 suggests that the exogenous kinase has not yet restored the extracts to M phase, in agreement with the extent of Cdc25 Ser287 and Cdk1 Tyr15 phosphorylations seen in A at t = 0.