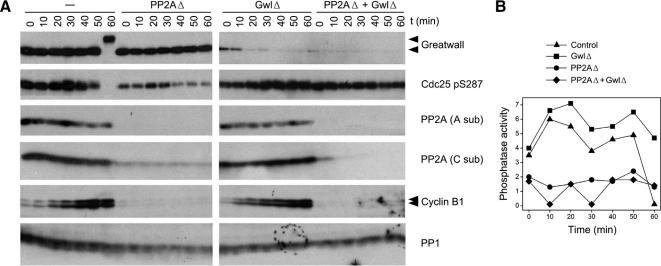
Figure 7.
Immunodepletion of PP2A removes most of the phosphatase activity directed against CDK sites. (A) Interphase cycling extracts were immunodepleted at 4°C for Gwl, PP2A, or both enzymes as indicated. PP2A immunodepletion was performed with the 6F9 mAb against the structural A subunit (Kremmer et al., 1997; Covance; no. MRT-204R) coupled to protein G-Sepharose (Zymed, South San Francisco, CA). The control extract was mock-depleted for both Gwl and PP2A, the GwlΔ extract was mock-depleted for PP2A, and the PP2AΔ extract was mock-depleted for Gwl. The extracts were then incubated at 22°C, and samples were removed for immunoblotting at the indicated times. Antibodies used for the Western blot included the 6F9 antibody for the PP2A A subunit and antibodies against the catalytic (C) subunits of PP1 and PP2A previously described by (Maton et al., 2005); these blots show the specificity of the immunodepletion for PP2A but not PP1. All samples depleted for PP2A remain in interphase because cyclin B1 is degraded and fails to accumulate for unknown reasons (see text). (B) Phosphatase assays of the samples shown in A, using substrate containing Thr311 of PP1γ. Although the PP2A depleted samples are clearly in interphase, they have on average <30% of the phosphatase activity of the corresponding nonPP2A-depleted controls.