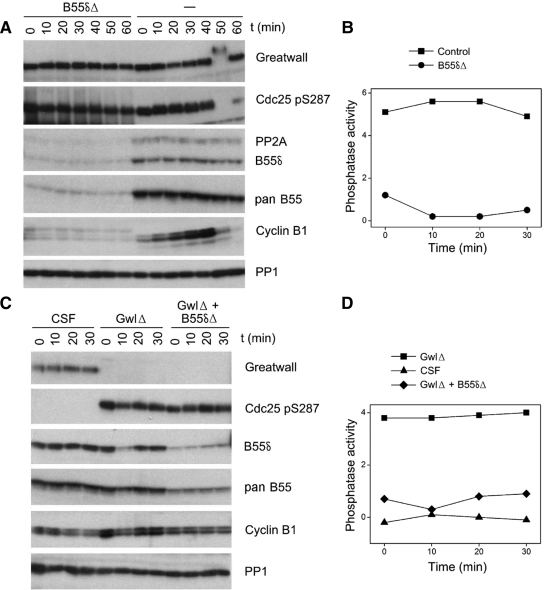
Figure 8.
PP2A/B55δ is a major constituent of the phosphatase directed against CDK phosphosites. (A) Cycling extracts were either mock-depleted (−) or depleted for B55δ (B55δΔ) for 1 h at 4°C and then incubated at 22°C starting at t = 0. The B55δ-depleted extracts were unable to enter M phase because cyclins were immediately degraded (see Discussion). (B) Phosphatase assays of the first four timed aliquots of the samples shown in A, using substrate containing Thr311 of PP1γ. Note that although both the control and B55δ-depleted extracts were in interphase during these times, the large majority of measured phosphatase activity was removed in the latter samples. (C) CSF extracts were either successively depleted for Gwl and B55δ (GwlΔ + B55δΔ), depleted for Gwl and then mock-depleted for B55δ (GwlΔ) or double mock-depleted (CSF). (D) Phosphatase assays of the samples shown in C, using substrate containing Thr311 of PP1γ. The phosphatase activity induced by Gwl depletion was substantially removed by subsequent B55δ immunodepletion. As judged by comparing the Western blot signals obtained with B55δ and pan B55 antibodies from the B55δ-depleted samples, B55δ accounted for more than 80% of the total B55 population in the extracts used in A and B and ∼60% of the B55 molecules in the extracts used in C and D.