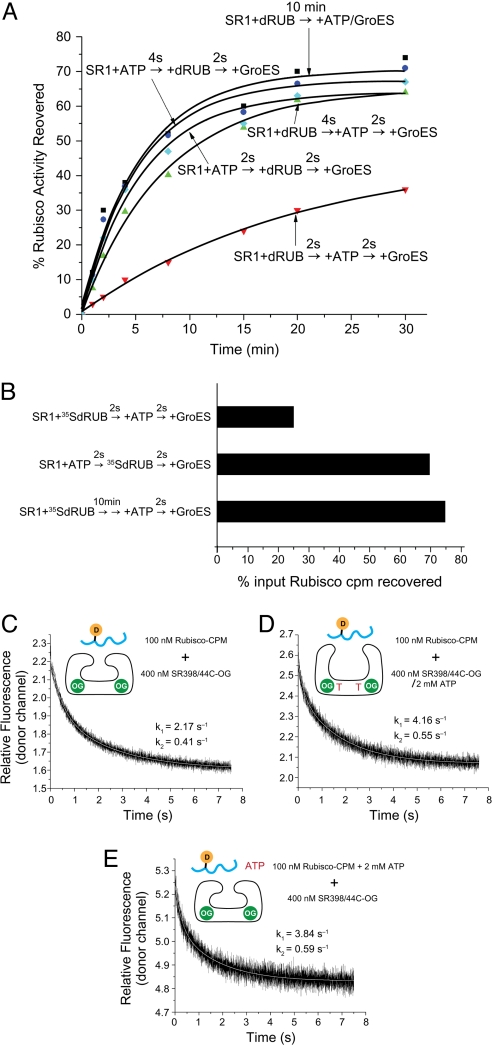
Fig. 3.
Binding of ATP before nonnative Rubisco improves folding yield by increasing the extent and rate of binding. (A) Recovery of Rubisco activity with different orders of addition. SR1 was incubated with ATP for 2 or 4 s before nonnative Rubisco (dRUB) was added; alternatively, nonnative Rubisco was added to SR1 first, followed by ATP 2 or 4 s later. For both orders, GroES was added 2 s later, and aliquots were removed at the indicated times for assay of Rubisco activity [cyan (2 s) and blue (4 s) for ATP first, and red (2 s) and green (4 s) for Rubisco first]. For comparison, a “standard” Rubisco refolding assay was carried out by incubating SR1 with nonnative Rubisco for 10 min before ATP and GroES were added together to start refolding (black symbols). (B) Extent of binding 35S-Rubisco to SR1 with different orders of addition. Experiments were carried out with the different orders of addition as in A, except that after adding GroES and then ADP-AlFx (to stabilize the ternary complex), the mixtures were chromatographed on a Superose 6 column and radioactivity of fractions was determined. Total radioactivity recovered at the elution position of the SR1/GroES/ADP-AlFx/Rubisco complex is reported as a percentage of the radioactivity loaded on the column. In identical analyses with 4-s intervals, both orders of addition produced ≈70% recovery, corresponding to the recovery of activity in A (not shown). (C) Rate of Rubisco binding to an SR1 ring in the absence of ATP, measured by FRET. Donor fluorescence of Rubisco-CPM after stopped-flow mixing with a hydrolysis-defective SR1 D398A molecule carrying the OG fluorophore on a substituted Cys-44. (D) Rate of Rubisco binding to SR398A in the presence of ATP (added before loading into the stopped-flow syringe). (E) Rate of Rubisco binding to an SR398A ring when added simultaneously with ATP.