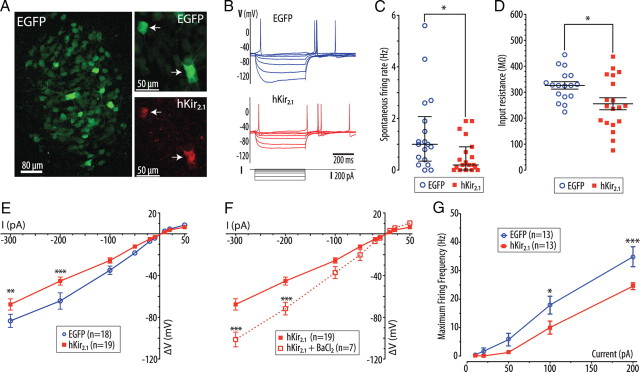
Figure 1.
Expression of hKir2.1 inhibits locus ceruleus neurons in vitro. A, Pontine slice culture (from P7 rat) after transduction with AVV-PRS-EGFP, showing EGFP expression in neurons of the LC. Right panels show LC neurons from a pontine slice culture transduced with AVV-PRS-EGFP and AVV-PRS-hKir2.1 (ratio 2:1) showing colocalization of EGFP and hKir2.1-immunoreactivity (white arrows indicate hKir2.1-positive neurons). B, Current clamp recordings from representative transduced LC neurons (control or hKir2.1-expressing) showing overlaid membrane potential responses to injected current pulses (+10 to −300 pA). C, LC neurons transduced with AVV-PRS-hKir2.1 had a reduced frequency of spontaneous action potential discharge (median 0.2 vs 1.0 Hz, interquartile range marked, Mann–Whitney test, *p < 0.05). D, Expression of hKir2.1 in LC neurons lowered their input resistances (256 ± 23 vs 327 ± 14 MΩ, unpaired t test, *p < 0.05). E, Examination of the current-voltage relationship showed that LC neurons expressing hKir2.1 had marked inward rectification (two-way ANOVA, **p < 0.01 and ***p < 0.001). F, This inward rectification was blocked by superfusion of barium (100 μm, two-way ANOVA, ***p < 0.001). G, Neurons were hyperpolarized to −75 mV (by DC current injection) to stop spontaneous action potential discharge. The maximum spike frequency response to injected current pulses (10–200 pA, 500 ms) was plotted showing that the LC neurons expressing hKir2.1 were less excitable (*p < 0.05 and ***p < 0.001, repeated measures ANOVA).