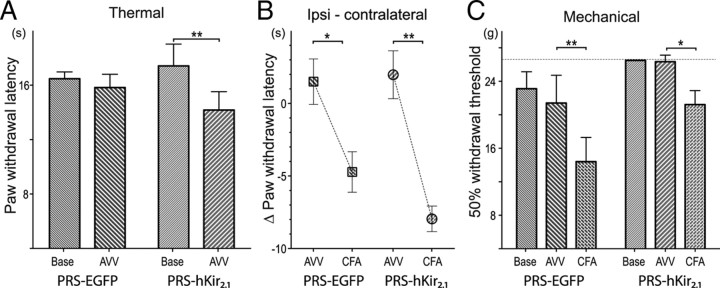
Figure 3.
Inhibition of pontospinal NAergic neurons causes thermal but not mechanical hyperalgesia. To examine the effect of inhibition of pontospinal NA neurons in a sensitized state we used the CFA inflammatory pain model in two groups of animals that had been transfected with AVV 10 d earlier (n = 7/group). A, Animals transduced with AVV-PRS-hKir2.1 showed thermal hyperalgesia on Hargreaves' testing [hKir2.1 17 ± 2 vs 14 ± 1 s (p < 0.05)] before CFA injection, this was not seen in the control animals (EGFP 16 ± 1 vs 16 ± 1 s (NS)] (two-way rmANOVA with Bonferroni post tests). B, To account for the change in baseline thermal nociception in the AVV-PRS-hKir2.1 group, the changes in ipsilateral hindpaw withdrawal latency after CFA was compared relative to the contralateral hindpaw. Injection of CFA produced thermal sensitization in both groups of animals [difference after CFA: EGFP, −6 ± 2 s, p < 0.01 vs hKir2.1, −10 ± 1 s, p < 0.001 (two-way rmANOVA with post hoc Bonferroni tests)]. Strikingly, the AVV-PRS-hKir2.1 group, which started from an already sensitized state, showed almost a twofold greater decrease in thermal withdrawal latency after CFA (hKir2.1 −8.0 s vs EGFP −4.7 s). C, Transduction with AVV had no significant effect on the mechanical withdrawal thresholds in either group. After CFA injection, both groups of animals showed significant sensitization (EGFP, p < 0.01; hKir2.1, p < 0.05), although the magnitude of this change was similar in both groups (EGFP, −7.0 g vs hKir2.1, −5.1 g).