Abstract
Background: Evidence from observational studies suggests that inadequate folate status enhances colorectal carcinogenesis, but results from some randomized trials do not support this hypothesis.
Objective: To assess the effect of folic acid supplementation on recurrent colorectal adenoma, we conducted a cost-efficient, double-blind, randomized trial among participants of 2 large prospective cohorts, the Health Professionals Follow-Up Study and the Nurses’ Health Study.
Design: Participants were randomly assigned to receive folic acid (1 mg/d) (n = 338) or placebo (n = 334) for 3–6.5 y. The primary endpoint was any new diagnosis of adenoma during the study period (May 1996–March 2004). Secondary outcomes were adenoma by site and stage and number of recurrent adenomas. Associations were also examined by plasma folate concentrations at baseline.
Results: Incidence of at least one recurrent adenoma was not significantly associated with folic acid supplementation [relative risk (RR): 0.82; 95% CI: 0.59,1.13; P = 0.22]. Among participants with low plasma folate concentrations at baseline (≤7.5 ng/mL), those randomly assigned to receive folic acid experienced a significant decrease in adenoma recurrence (RR: 0.61; 95% CI: 0.42, 0.90; P = 0.01), whereas for subjects with high folate concentrations at baseline (>7.5 ng/mL), supplemental folic acid had no significant effect (RR: 1.28; 95% CI: 0.82, 1.99; P = 0.27, Pinteraction = 0.01). Contrary to findings from another clinical trial, there was no evidence for an increased risk of advanced or multiple adenomas.
Conclusions: Our results do not support an overall protective effect of folic acid supplementation on adenoma recurrence. Folic acid supplementation may be beneficial among those with lower folate concentrations at baseline. This trial was registered at clinical trials.gov as NCT00512850.
INTRODUCTION
Animal and human studies have suggested that folate, which is involved in DNA synthesis and methylation, may play a protective role in colorectal carcinogenesis (1, 2). Results from clinical trials that have examined the association between folic acid (FA) supplementation and recurrence of colorectal adenoma are inconsistent. In one recent small clinical trial that included 137 participants (94 of whom were included in the main analysis) who were randomly assigned to receive either 5 mg FA or placebo daily for an average of 3 y, FA supplementation appeared to lower the mean number of adenomas (3). In another small clinical trial (4) that included 60 subjects, 13% of participants in the FA-supplemented group were found to have recurrent adenomas after 24 mo compared with 28% in the placebo group; however, differences were not statistically significant.
On the contrary, 2 recent large clinical trials, the Aspirin/Folate Polyp Prevention Study (AFPPS) (5) and the United Kingdom Colorectal Adenoma Prevention (ukCAP) trial (6) showed little evidence for a protective effect of FA supplementation on recurrent adenomas, and in 1 of these 2 trials, FA supplementation was associated with higher risk of advanced and multiple adenomas (5). This article reports on another randomized trial of FA supplementation (1 mg/d) and recurrent adenoma among participants of 2 large prospective cohorts, the Health Professionals Follow-Up Study (HPFS) and the Nurses’ Health Study (NHS). In both cohorts, previous analyses had suggested that higher folate consumption was associated with a lower risk of colorectal adenoma (7).
SUBJECTS AND METHODS
Study cohort
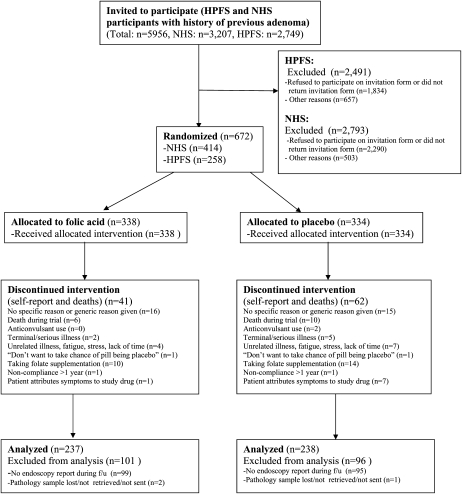
The CONSORT (Consolidated Standards of Reporting Trials) flowchart (8) for the NHS/HPFS Folic Acid Prevention Trial is shown in Figure 1. Because adenoma recurrence trials are typically expensive to conduct, we designed a double-blind randomized intervention trial that could be conducted efficiently and at low cost. We used the resources of 2 large US cohorts, the HPFS and the NHS, and were able to absorb much of the costly components of an intervention trial, such as recruitment, follow-up, and obtaining medical records, into already funded cohort activities. This trial was approved by the Human Subjects Committee of the Brigham and Women’s Hospital and the Human Subjects Committee of the Harvard School of Public Health.
FIGURE 1.
The CONSORT (Consolidated Standards of Reporting Trials) flowchart. The Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) Folic Acid Prevention Trial. f/u, follow-up.
The NHS and HPFS cohorts have been described in detail elsewhere (9, 10). In brief, the NHS cohort was started in 1976 and included 121,700 female US nurses aged between 30 and 55 y. The baseline questionnaire that was mailed to the women asked them to report on lifestyle and medical history. In 1980, a food-frequency questionnaire (FFQ) was included (10). The HPFS cohort was initiated in 1986 when 51,129 US male health professionals aged between 40 and 75 y responded to a mailed questionnaire that inquired about lifestyle and medical history and a 136-item FFQ (9). Every 2 y, participants received another questionnaire that requested updated lifestyle and medical history, and every 4 y the questionnaire included an FFQ to update the participants’ diet (9, 10). Only participants from both cohorts with colorectal adenoma that was confirmed by medical records and pathology reports were invited to participate in the trial. Those initial adenomas were ascertained as part of our regular cohort disease follow-up.
Participants from the NHS and HPFS cohorts were eligible for this trial if they met following eligibility criteria: 1) had a history of colorectal adenoma confirmed by medical record review, 2) planned on having an another endoscopy ≤4 y after initiation of trial, 3) agreed not to take multivitamins or any other supplements containing FA during the course of the trial, and 4) were cancer free at the time of randomization except for early-stage prostate or breast cancer or nonmelanoma skin cancer. Because FA is a water-soluble vitamin, toxicity from FA supplementation at the dose administered in our trial (1 mg/d) is considered to be low, with the only possible adverse effect being the “masking of a vitamin B-12 deficiency” (11–13). Therefore, we only included participants in our study who were not deficient in vitamin B-12 (defined as <200 pg/mL or as 200–299 pg/mL with methylmalonic acid concentrations >32 μg/L) on the basis of a screening blood sample. In addition, participants were ineligible if they were presently taking multivitamin, FA, or vitamin B-12 supplements for a diagnosed vitamin deficiency or were following their physicians’ recommendation that they take multivitamins, FA, or vitamin B-12 for any indication; had a diagnosis of homocysteinemia or pernicious anemia; had a history of gastrectomy or a gastrointestinal disorder that could lead to vitamin B-12 deficiency or a history of total colectomy; or had a diagnosis of cirrhosis, pancreatitis, or pancreatectomy. We also excluded participants who were taking methotrexate or anticonvulsants because FAs may interact with these drugs (11, 13). The safety and efficacy of FA supplementation is reviewed elsewhere (11, 13).
Randomization and intervention
A total of 672 participants aged between 50 and 78 y were ultimately assigned to receive either FA (1 mg/d; n = 338) or placebo (n = 334). This trial did not have a run-in period to assess compliance with pill-taking; however, we collected adherence blood samples (second blood samples) at approximately the midtrial point (also see Adherence samples below) to compare folate concentrations measured at baseline with those measured at midtrial. Randomization by a random-number generator was performed at the Investigational Drug Service at the Brigham and Women’s Hospital Pharmacy Department. Similar-appearing FA and placebo pills were provided by the pharmaceutical company Lederle (now Wyeth Pharmaceuticals, Collegeville, PA) and were shipped directly from the pharmaceutical company to the Investigational Drug Service, which was responsible for mailing the pills to the participants. Each shipment provided participants with pills for 6 mo. Participants as well as study investigators were blinded to their study arm. Included in the first shipment and then every following year were short questionnaires that also included a question regarding the date of the participant’s most recent lower bowel endoscopy.
Study period and extension group
Randomization and all mailings for this trial were conducted in 9 groups, with each consisting of 45–124 participants. The first group received their first shipment of pills in May 1996, and the final group received their first shipment of pills in October 1999. This period encompassed the implementation of FA fortification of flour in the United States (14). The supplement duration was originally designed to be 3 y; however, during the trial, the study period was extended for a total of 5–6.5 y. The decision to extend the trial period was based on findings from the NHS cohort that suggested that longer duration of FA supplementation may be important to convey protection against colorectal cancers (15). Three hundred twenty-seven participants (49%) gave consent to have their study period extended (150 placebo and 177 FA). Because we counted all endoscopies up to 12 mo after completion of the trial as endoscopies performed during the study period (see below), the entire study period encompassed May 1996 to March 2004 (mean ± SD length of follow-up: 64.1 ± 17.1 mo).
Follow-up endoscopies and confirmation of recurrent adenoma
All follow-up for this trial was conducted through the postal system. Participants who reported a recent endoscopy on the trial questionnaire were mailed a consent form to get permission to obtain and review their medical records pertaining to large bowel endoscopic procedures from their doctor’s office and/or the hospital. After receiving signed consent forms from the participants, one trial investigator reviewed the medical records and extracted information on site, size, histology, and number of recurrent adenomas. We only counted endoscopies performed ≥3 mo after initiation of trial and ≤12 mo after completion of the trial as endoscopies performed during the study period. During the follow-up period, 478 participants (71%) had ≥1 endoscopy, 97 (14%) had 2 endoscopies, and 7 participants (1%) had 3 endoscopies. For 3 of these 478 participants, a diagnosis of adenoma could not be made because the specimen was lost, was not retrieved, or was not sent to the pathologist. Therefore, a total of 475 participants were included in our main analysis. The vast majority of those 475 participants had received at least one colonoscopy during the trial, and 2% (10 participants) had only received a sigmoidoscopy during the trial. Of those 475 participants, 277 (58%) had agreed to extend their treatment.
Dropouts (self-reported)
During the course of the trial, 100 participants or their next of kin contacted us to inform us that they did not want to continue treatment (n = 87) or that the participant had died during the follow-up (n = 13). In addition, during our regular death follow-up in our cohorts, 3 more participants were found to have died during the trial, which brought the total number of reported dropouts during the trial to 103. We were still able to receive endoscopy reports from 48 of those 103 participants, including 3 who subsequently died but who had received an endoscopy.
Adherence samples
About halfway through the trial, participants in the first 8 groups were asked to provide a second blood sample to assess adherence to the treatment (due to limited resources, group 9 was not invited to provide a second sample). A total of 484 participants (200 HPFS and 284 NHS) returned an adherence sample (77% of invited participants). Of the 475 participants included in the main analysis, 375 (79%) had provided adherence samples. All samples were stored at −70°C in liquid nitrogen.
Laboratory analysis
Baseline and adherence samples were analyzed in the laboratory of Jacob Selhub at the Jean Mayer US Department of Agriculture Human Nutrition Research Center on Aging at Tufts University, Boston, MA. Folate and vitamin B-12 concentrations at baseline were determined by using a radioassay kit (Ciba-Corning, Walpole, MA) as described in another publication (16). Folate concentrations for the compliance samples were determined by a boil, liquid-phase, competitive, ligand-labeled protein binding chemiluminescent commercial kit procedure on the IMMULITE 1000 (IMMULITE/IMMULITE 1000 Folic Acid, document PILKF; Siemens Healthcare Diagnostics, Deerfield, IL).
Statistical analysis
Statistical analysis was performed by using the intention-to-treat approach. Baseline characteristics were compared by chi-square test to compare frequencies of baseline characteristics between the FA-supplemented group and placebo group. To assess the association between FA supplementation and the risk of at least one recurrent adenoma, we estimated risk ratios by using a generalized linear model with a natural logarithm link function and Poisson distributed errors, which were adjusted for over- and underdispersion. The “basic model” included age at start of trial (continuous), sex, extension group (yes or no), and time between start of trial and last follow-up endoscopy. The “final multivariate model” included all variables from the basic model as well as the following: body mass index (BMI) (continuous); physical activity (continuous); pack-years of smoking (continuous); aspirin use (<2 or ≥2 tablets/wk); intakes of folate, alcohol, and red and processed meat (all as continuous variables); family history of colorectal cancer (yes or no); and number (1, 2, or ≥3) and stage of adenoma (early or advanced) before randomization. Because estimates from the basic and the final model were similar, only estimates from the basic model are presented in this article. To assess associations between number of adenomas and treatment group, we used the generalized estimating equations model with a natural logarithm link function and a Poisson distribution with SAS PROC GENMOD (SAS OnlineDoc 9.1.2; SAS Institute Inc, Cary, NC).
Given the strong a priori evidence of an interaction between folate and alcohol, stratification by baseline folate and alcohol status were 2 important prespecified interactions to be examined (1). Therefore, associations were examined after stratification by folate concentration at baseline (below and above median of 7.5 ng/mL), alcohol intake at baseline (below and above median of 5.6 g/d), and combinations of folate concentrations and alcohol intake. We also examined interactions by age (≤66 or >66 y), smoking status (ever or never), BMI (in kg/m2; ≤25 or >25) and aspirin use (<2 or ≥2 tablets/wk). P values for interactions were assessed by including a multiplicative interaction term of intervention status and the variable of interest as a binary variable. A Wald test of the coefficient for the cross-term product was used to obtain P values for interaction.
Additionally, we examined associations between FA supplementation and secondary outcome measures—ie, adenoma subsite (proximal or distal), size [small (<1cm) or large (≥1 cm)], and stage (early: small and tubular; advanced: large or any villous histology, high-grade dysplasia, or colorectal cancer).
We also conducted several sensitivity analyses. First, we examined associations after excluding those participants who had notified us that they wanted to drop out or who had died during the study but for whom we had received an endoscopy report during the study period. Second, because we did not specifically require participants to undergo an endoscopy before randomization, we also restricted analyses to participants who, according to information obtained from medical records, had undergone an endoscopy ≤2 y before the start of the trial. Third, to assess whether associations differ by duration of exposure to FA, we also analyzed associations by time between start of trial and first endoscopy (≤24 compared with >24 mo).
RESULTS
Because of the implementation of FA supplementation (14) during the course of the trial, over time folate concentrations (baseline compared with midtrial) increased significantly in all participants with available measurements on folate at baseline and midtrial (n = 480). However, the overall increase in folate concentration was more pronounced in the FA group than in the placebo group [mean ± SD baseline compared with midtrial: 9.3 ± 6.2 compared with 17.0 ± 7.9 ng/mL (P < 0.05) and 9.7 ± 6.8 compared with 39.2 ± 24.8 ng/mL in placebo and FA groups, respectively (P < 0.05)], which suggests reasonable pill compliance among participants who had donated 2 blood samples.
Most baseline characteristics did not differ significantly by intervention group (Table 1). Participants in the FA group were less likely to have a family history of colorectal cancer than participants in the placebo group (total study population: P = 0.06; participants included in main analyses; P = 0.33). More importantly, baseline characteristics of participants who were included in the main analyses—ie, participant who had an endoscopy during the follow-up period (n = 475)—were similar to all randomly assigned participants (n = 672). In addition, among the 194 participants for whom we did not receive a follow-up endoscopy (FA: n = 99; placebo: n = 95; 3 participants did receive an endoscopy during the follow-up but were excluded from analyses due to insufficient information), baseline characteristics such as age or severity of adenoma at baseline—ie, factors that may have affected the likelihood of a follow-up endoscopy—did not differ considerably by treatment group (mean age at baseline: 65.7 and 66.4 y in FA and placebo groups, respectively; P = 0.35; advanced adenoma at baseline: 44.4% and 48.4% in FA and placebo groups, respectively; P = 0.46).
TABLE 1.
Baseline characteristics of participants by intervention group: the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) Folic Acid Prevention Trial1
| All participants |
Participants with follow-up endoscopies (included in main analyses) |
|||
| Characteristics | Placebo (n = 334) | Folic acid (n = 338) | Placebo (n = 238) | Folic acid (n = 237) |
| Study cohort [n (%)] | ||||
| HPFS men | 126 (38) | 132 (39) | 89 (37) | 90 (38) |
| NHS women | 208 (62) | 206 (61) | 149 (63) | 147 (62) |
| Age at start of pill-taking (y)2 | 65.7 ± 6.53 | 64.9 ± 6.7 | 65.4 ± 6.3 | 64.5 ± 6.9 |
| BMI (kg/m2)2 | 25.8 ± 3.8 | 25.7 ± 3.9 | 25.5 ± 3.4 | 25.6 ± 3.7 |
| Physical activity (MET-h/wk)2 | 23.7 ± 26.8 | 24.0 ± 22 | 23.7 ± 24 | 23.7 ± 20.9 |
| Intake2 | ||||
| Red meat (servings/d) | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| Processed meat (servings/d) | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.3 ± 0.2 | 0.3 ± 0.2 |
| Methionine (g/d) | 1.9 ± 0.3 | 1.9 ± 0.3 | 1.9 ± 0.3 | 1.9 ± 0.3 |
| Total folate (μg/d) | 446 ± 177 | 428 ± 163 | 441 ± 166 | 425 ± 164 |
| Dietary folate (μg/d) | 319 ± 84.3 | 325 ± 89.1 | 324 ± 77.7 | 323 ± 92.6 |
| Alcohol (g/d) | 9.6 ± 12.5 | 10.6 ± 13.4 | 9.8 ± 12.8 | 10.7 ± 13.9 |
| Energy (kcal/d) | 1819 ± 480 | 1827 ± 477 | 1832 ± 482 | 1807 ± 460 |
| Total calcium (mg/d) | 981 ± 353 | 942 ± 312 | 950 ± 324 | 941 ± 306 |
| Total vitamin D (IU/d) | 375 ± 201 | 351 ± 182 | 374 ± 199 | 343 ± 179 |
| Family history of colorectal cancer [n (%)]2 | 106 (32) | 85 (25) | 76 (32) | 66 (28) |
| Current smoking [n (%)]2 | 24 (7) | 23 (7) | 15 (6) | 18 (8) |
| Current use of aspirin ≥2/wk [n (%)]2 | 138 (41) | 145 (43) | 99 (42) | 103 (43) |
| Multivitamin use before randomization [n (%)]2 | 139 (42) | 134 (40) | 101 (42) | 98 (41) |
| Stage of adenoma before randomization [n (%)]4 | ||||
| Early adenoma (small/tubular) [n (%)] | 125 (37) | 121 (36) | 92 (39) | 91 (38) |
| Advanced adenoma (large or villous histology or high-grade dysplasia) [n (%)] | 146 (44) | 146 (43) | 100 (42) | 100 (42) |
| Not specified/missing [n (%)] | 63 (19) | 71 (21) | 46 (19) | 46 (19) |
| No. of adenomas before randomization [n (%)]4 | ||||
| 1 | 218 (65) | 208 (62) | 154 (65) | 148 (62) |
| 2 | 42 (13) | 47 (14) | 32 (13) | 29 (12) |
| ≥3 | 22 (7) | 28 (8) | 12 (5) | 20 (8) |
| Not specified/missing | 52 (16) | 55 (16) | 40 (17) | 40 (17) |
| Folate concentration at baseline (ng/mL)5 | 9.3 | 9.7 | 9.1 | 9.2 |
| Total no. of endoscopies during trial [n (%)]6 | ||||
| 0 | 95 (28) | 99 (29) | N/A | N/A |
| 1 | 182 (54) | 192 (57) | 182 (76) | 190 (80) |
| 2 | 52 (16) | 45 (13) | 51 (21) | 45 (19) |
| 3 | 5 (2) | 2 (0.5) | 5 (2) | 2 (1) |
| Time between start of trial and endoscopy (mo) | ||||
| First endoscopy | N/A | N/A | 24 ± 15.3 | 26 ± 15.2 |
| Second endoscopy | N/A | N/A | 50 ± 17.9 | 53 ± 15.0 |
| Third endoscopy | N/A | N/A | 47 ± 6.6 | 38 ± 7.8 |
MET, metabolic equivalent task; N/A, not applicable. P values were calculated by using Wilcoxon’s rank-sum test for continuous variables and chi-square or Fisher’s exact test, if applicable, for categorical variables (all P values > 0.05).
Information obtained from cohort questionnaires ≤2 y before start of intervention or, if missing, carried forward from most recent cohort questionnaire.
Mean ± SD (all such values).
Due to missing information on either number or histology of prior adenoma, percentages do not add up to 100.
Plasma folate measurement for one participant in the HPFS is missing.
For “all participants,” all endoscopies were counted (n = 478) that were performed at least 3 mo after start of trial and up to 1 y after completion of trial; for “participants included in the main analyses,” 3 endoscopies were not counted for those participants’ whose tissue was not retrieved, was lost, or was not sent to pathology, and those participants were excluded from the main analyses.
The treatment groups did not differ appreciably with regard to occurrence of cardiovascular disease or noncolorectal cancers after randomization, (Table 2), but the number of deaths was slightly but nonsignificantly higher in the placebo compared with the FA group. Among the 194 participants without a follow-up endoscopy, more participants in the placebo group had died (n = 13; 13.7%) than those in the FA group (n = 5; 5.1%) (P = 0.04), and more participants in the placebo group developed cancers (n = 9; 9.5%) than those in the FA group (n = 3; 3%) (P = 0.06). The respective numbers and percentages for cardiovascular events were as follows: fatal cardiovascular disease: 0 and 3 (3.2%) for FA and placebo groups, respectively; nonfatal myocardial infarction: 1 (1%) and 0 for FA and placebo groups, respectively; and nonfatal stroke: 0 and 3 (3.2%) for FA and placebo groups, respectively.
TABLE 2.
Occurrence of deaths, cancers, and cardiovascular disease after randomization in all participants by intervention group: the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) Folic Acid Prevention Trial1
| Event | Placebo (n = 334) | Folic acid (n = 338) | P value |
| Deaths2 | 15 (4) | 7 (2) | 0.08 |
| All cancers | 24 (7) | 24 (7) | 0.97 |
| Colorectal cancer3 | 3 (0.9) | 1 (0.3) | 0.37 |
| Lung cancer | 3 (0.9) | 4 (1) | 1.00 |
| Breast cancer in women | 6 (2) | 5 (1) | 0.75 |
| Prostate cancer (excluding stage A1) | 6 (2) | 5 (1) | 0.75 |
| Nonfatal myocardial infarction4 | 1 (0.3) | 6 (2) | 0.12 |
| Fatal cardiovascular disease45 | 3 (0.9) | 0 | 0.12 |
| Nonfatal stroke4 | 3 (0.9) | 4 (1) | 1.00 |
| Fatal stroke4 | 0 | 0 |
All values are n; percentages in parentheses. Only cancer and cardiovascular events that occurred after randomization and up to 1 y after completion of trial are included. P values were calculated by using a chi-square test or Fisher’s exact test, if applicable.
Includes deaths after randomization and up to 1 y after completion of trial.
One case of squamous cell histology.
Frequencies for nonfatal myocardial infarction and fatal cardiovascular disease and for nonfatal stroke and fatal stroke are mutually exclusive; if both a nonfatal and fatal event occurred after randomization, the fatal event was chosen. However, cancer and cardiovascular events were not considered mutually exclusive.
Fatal cardiovascular disease includes International Classification of Diseases and Related Health Problems, Ninth Edition (ICD-9) codes 410–414 for participants in the HPFS and ICD-9 codes 410 and 412 for participants in the NHS.
Most adenomas that were detected during follow-up were proximal and early adenomas (Table 3). FA supplementation was not significantly associated with the incidence of at least one adenoma (RR: 0.87; 95% CI: 0.65, 1.16) (Table 4). An inverse association between FA supplementation and recurrent distal and early-stage adenoma diagnosed on the first endoscopy was suggested, but none of the associations reached statistical significance (Table 4). Randomization to FA did not significantly influence the risk of advanced adenomas (RR: 1.03; 95% CI: 0.53, 1.98). When we examined the number of adenomas detected on all endoscopies during the trial period, no association between FA supplementation and number of recurrent adenomas (per one adenoma increase: RR: 0.85; 95% CI: 0.61, 1.17; P = 0.31) was found. When we examined associations by time between start of trial and first endoscopy (≤24 compared with >24 mo), associations between recurrent adenoma and FA supplementation were similar (data not shown).
TABLE 3.
Adenoma characteristics in participants with follow-up endoscopies and recurrent adenoma by intervention group: the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) Folic Acid Prevention Trial1
| Adenoma characteristics | Placebo (n = 72) | Folic acid (n = 62) | P value |
| Location of adenoma2 | |||
| Rectum only | 8 (11) | 3 (5) | |
| Distal only | 19 (26) | 18 (29) | |
| Proximal only | 34 (47) | 36 (58) | 0.38 |
| ≥2 Locations | 10 (14) | 4 (6) | |
| Not specified | 1 (1) | 1 (2) | |
| Histologic type3 | |||
| Tubular | 44 (61) | 38 (61) | |
| Tubular–villous | 10 (14) | 7 (11) | |
| Villous | 1 (1) | 1 (2) | 0.97 |
| High-grade dysplasia/colorectal cancer | 2 (3) | 1 (2) | |
| Not specified | 15 (21) | 15 (24) | |
| Size4 | |||
| Small | 55 (76) | 45 (73) | |
| Large | 11 (15) | 10 (16) | |
| Not specified | 6 (8) | 7 (11) | 0.83 |
| Stage5 | |||
| Early (small and tubular) | 42 (58) | 30 (48) | |
| Advanced (large or tubular–villous or villous or high-grade dysplasia or colorectal cancer) | 17 (24) | 16 (26) | |
| Not specified | 13 (18) | 16 (26) | 0.45 |
| No. of adenomas6 | |||
| 1 | 53 (74) | 48 (77) | |
| ≥2 | 18 (25) | 13 (21) | |
| Not specified | 1 (1) | 1 (2) | 0.84 |
| Time between start of trial and first adenoma (mo) | 29.4 | 35.2 | 0.10 |
All values are n; percentages in parentheses. P values were calculated by using chi-square test or Fisher’s exact test, if applicable.
Denotes location of any adenoma.
Denotes worst histology of any adenoma.
Denotes largest size detected.
Denotes worst stage diagnosed.
Sum of all adenomas diagnosed during the follow-up period regardless of frequency of endoscopy.
TABLE 4.
Risk ratios (RRs) for recurrent adenoma by intervention group: the Nurses’ Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) Folic Acid Prevention Trial1
| First endoscopy |
All endoscopies (first–third) |
|||||||
| Outcome | Placebo (n = 238) | Folic acid (n = 237) | RR (95% CI) | P value | Placebo (n = 238) | Folic acid (n = 237) | RR (95% CI) | P value |
| no. cases/total n | no. cases/total n | no. cases/total n | no. cases/total n | |||||
| At least one adenoma2 | 64/238 | 52/237 | 0.82 (0.59, 1.13) | 0.22 | 72/238 | 62/237 | 0.87 (0.65, 1.16) | 0.33 |
| Subsite3 | ||||||||
| At least one proximal adenoma4 | 36/224 | 35/226 | 0.97 (0.63,1.49) | 0.88 | 43/228 | 40/228 | 0.93 (0.63, 1.38) | 0.71 |
| At least one distal adenoma | 25/237 | 15/236 | 0.60 (0.32,1.11) | 0.11 | 28/237 | 21/236 | 0.74 (0.43, 1.27) | 0.28 |
| Size of adenoma3 | ||||||||
| Small | 50/234 | 37/232 | 0.74 (0.50, 1.09) | 0.13 | 55/232 | 45/230 | 0.82 (0.58, 1.17) | 0.27 |
| Large | 10/234 | 10/232 | 1.07 (0.45, 2.51) | 0.88 | 11/232 | 10/230 | 0.97 (0.42, 2.24) | 0.94 |
| Stage3 | ||||||||
| Early | 39/228 | 25/225 | 0.64 (0.40, 1.03) | 0.06 | 42/225 | 30/221 | 0.71 (0.46, 1.10) | 0.13 |
| Advanced | 15/228 | 15/225 | 1.08 (0.54, 2.16) | 0.82 | 17/225 | 16/221 | 1.03 (0.53, 1.98) | 0.94 |
| No. of adenomas (first endoscopy only) | ||||||||
| 1 | 50/237 | 42/236 | 0.84 (0.58, 1.22) | 0.36 | N/A | N/A | ||
| ≥2 | 13/237 | 9/236 | 0.72 (0.31, 1.67) | 0.45 | ||||
RRs were calculated by using a generalized linear model with a natural logarithm link function and Poisson distributed errors, which were adjusted for over- and underdispersion. Models included age at start of trial, sex, length of trial (3 compared with ≤6.5 y), and time between start of trial and last endoscopy (mo).
Three participants were excluded from analysis because their tissue was not retrieved, lost, or not sent to pathology.
Denominator does not include participants with missing information for site, size, or stage.
Denominator for proximal adenoma analysis is lower because only participants who had a complete colonoscopy were included. For the “first endoscopy” analyses, we excluded 5% of participants; for the “all endoscopy” analyses, we excluded 4% of participants.
Associations between FA supplementation and total adenoma recurrence did not appear to differ considerably by age, aspirin use, smoking status, and alcohol intake (Table 5) (all Pinteraction > 0.05). Among participants with low BMI (≤25), some evidence for a marginally significant inverse association between FA supplementation and recurrent adenoma was found, whereas for subjects with a high BMI (>25), supplemental FA was not associated with recurrent adenoma. However, Pinteraction was not statistically significant (Pinteraction = 0.11). A significant inverse association between FA supplementation and recurrent adenoma was observed between those with low plasma folate concentrations at baseline (RR: 0.61; 95% CI: 0.42, 0.90), whereas no association was found between those with high plasma folate concentrations at baseline (RR: 1.28; 95% CI: 0.82, 1.99; Pinteraction = 0.01). Stronger inverse associations were observed for participants who had both low plasma folate concentrations and high alcohol intake. Among participants with both low folate concentrations and low alcohol intake at baseline, however, there was no association between FA supplementation and adenoma recurrence (RR: 0.84; 95% CI: 0.47, 1.49; P = 0.54), but sample size was limited (n = 35 cases). Associations between FA supplementation and recurrent adenoma were similar after exclusion of participants who had informed us that they wished to stop taking pills or who had died during the study but for whom a report on endoscopy during the trial was obtained (n = 48) (data not shown)
TABLE 5.
Risk ratios (RRs) for recurrent adenoma by intervention group: stratified analyses of the Nurses' Health Study (NHS) and Health Professionals Follow-Up Study (HPFS) Folic Acid Prevention Trial1
| At least one adenoma |
||||
| Stratification variable | Placebo | Folic acid | Risk ratio (95% CI) | P value |
| no. cases/total n | no. cases/total n | |||
| Age | ||||
| ≤66 y | 42/130 | 33/133 | 0.79 (0.54, 1.17) | 0.24 |
| >66 y | 30/108 | 29/104 | 1.00 (0.64, 1.55) | 0.99 |
| Aspirin | ||||
| <2 tablets/wk | 38/139 | 33/134 | 0.88 (0.59, 1.32) | 0.54 |
| ≥2 tablets/wk | 34/99 | 29/103 | 0.85 (0.56, 1.30) | 0.46 |
| BMI | ||||
| ≤25 kg/m2 | 36/115 | 26/120 | 0.66 (0.43, 1.03) | 0.06 |
| >25 kg/m2 | 36/123 | 36/117 | 1.08 (0.73, 1.59) | 0.72 |
| Smoking status | ||||
| Never | 27/96 | 24/88 | 1.03 (0.64, 1.66) | 0.89 |
| Ever | 45/141 | 37/145 | 0.79 (0.54, 1.14) | 0.21 |
| Folate concentrations at baseline | ||||
| ≤7.5 ng/mL | 42/113 | 32/139 | 0.61 (0.42, 0.90) | 0.01 |
| >7.5 ng/mL | 30/124 | 30/98 | 1.28 (0.82, 1.99) | 0.27 |
| Alcohol intake | ||||
| ≤5.6 g/d | 31/117 | 33/122 | 1.00 (0.65, 1.53) | 1.00 |
| >5.6 g/d | 41/121 | 29/115 | 0.76 (0.51, 1.13) | 0.17 |
| Combination alcohol intake/folate baseline | ||||
| ≤5.6 g/d and gt7.5 ng/mL | 14/62 | 15/53 | 1.23 (0.64, 2.37) | 0.53 |
| >5.6 g/d and le7.5 ng/mL | 25/59 | 14/70 | 0.49 (0.28, 0.84) | 0.009 |
RRs were calculated by using a generalized linear model with a natural logarithm link function and Poisson distributed errors, which were adjusted for over- and underdispersion. Models included age at start of trial, sex, length of trial (3 compared with ≤6.5 y), and time between start of trial and last endoscopy (mo). All P values for interaction were >0.05, except for folate at baseline (P = 0.01) and combination alcohol/folate at baseline (P = 0.03).
The association between FA supplementation and risk of recurrent adenoma did not differ appreciably after restricting analysis to participants who, through information obtained from medical records, had had an endoscopy ≤2 y before the start of the trial (RR: 0.94; 95% CI: 0.53, 1.67; placebo: n = 89, 20 cases; folate: n = 100, 19 cases).
DISCUSSION
Our results do not suggest a protective effect of 1 mg FA supplementation/d on the overall risk of recurrent adenoma, however, FA supplementation lowered the risk of recurrent adenoma in participants with low plasma folate concentrations at baseline, especially those with a combination of low plasma folate and high alcohol intake (a folate antagonist).
Contrary to results from 2 smaller clinical trials (3, 4), the AFPPS trial (5) found no evidence for a protective association between FA supplementation (1 mg/d) and recurrent adenoma after 3 y of intervention. However, after an additional 3–5 y of intervention, risk of advanced and higher numbers of adenomas was higher in participants randomly assigned to the FA supplement group, and a higher incidence of prostate and other noncolorectal cancers in the FA group was found. In the ukCAP trial (6), no association between FA supplementation (500 μg/d) and recurrent adenoma was observed after an average of 3 y. However, in contrast to the AFPPS trial, FA supplementation was not associated with increased risk of advanced adenoma, and number of adverse events including noncolorectal cancers did not differ by treatment group. The lower dose of FA and the shorter period of the intervention in the ukCAP trial (6) compared with the AFPPS trial (5) may, in part, explain the difference in findings between these 2 trials. Furthermore, unlike in the United Kingdom, in the United States FA fortification was mandated and implemented (in January 1998) during the conduct of the AFPPS (14).
Given the strong a priori evidence of an interaction between folate and alcohol, stratification by baseline folate and alcohol status—2 important prespecified interactions—were examined (1). Contrary to the AFPPS trial, we found some benefit of FA supplementation on adenoma recurrence among those with lower folate concentrations at baseline, especially among those with both high alcohol intake and low folate concentrations. One possible explanation could be the existence of a nonlinear dose-response relation between FA and adenoma—ie, FA supplementation may only benefit those with low folate status at baseline. However, due to the implementation of FA fortification during the course of the trial (14), folate concentrations increased significantly in the placebo group regardless of folate status at baseline (participants included in the main analysis only: within the placebo arm, the mean midtrial folate concentration was 15.7 ng/mL in the low baseline folate group and 17.9 ng/mL in the high baseline folate group), which limited our ability to explore this hypothesis further. Another possible explanation for our findings is the existence of a “folate-independent pathway,” ie, participants with high baseline folate who still developed adenoma (only participants with a history of colorectal adenoma were included in this trial) might be cases whose adenoma developed through a folate-independent pathway, whereas some participants with low folate at baseline, especially those with high alcohol intake, may benefit from FA supplementation. If confirmed, this observation could enhance our understanding of the role of folate in colorectal carcinogenesis. However, in consideration of the small number of cases in these 2 groups as well as the lack of association observed in the AFPPS trial, chance could also explain our findings.
There are some limitations inherent to our study design that require further discussion. First, contrary to the AFPPS trial, the specimens in our trial were not reviewed by a central pathologist. In our trial, outcome was based on a review of pathology results, which may have contributed to some misclassification of outcome, especially with regard to stage of disease. Second, during the time the AFPPS and this trial were conducted, FA fortification was mandated in the United States (14). However, the amount of additional FA received through fortified foods was probably considerably lower than the dose administered in both trials (≈100 compared with 1000 μg/d) (17). In our trial, participants’ folate concentrations increased significantly over time, but on average the increase was more pronounced in the FA than in the placebo group. Further support that fortification probably did not considerably affect the results from the 2 US trials is provided by the lack of association between FA supplementation and recurrent adenoma in the ukCAP trial in which FA fortification was not mandated and a lower dose of FA was administered.
Third, unlike the ukCAP and the AFPPS trial, our trial was conducted entirely through the postal system, and the proportion of randomized participants who received at least one endoscopy during the study period was lower (71%) than in the APPS trial (97%) (5) or the ukCaP trial (91%) (6). However, this issue would only play a role if the likelihood of getting a follow-up endoscopy would differ by treatment group. Because participants and their physicians were blinded to treatment status, it is conceivable that the likelihood of receiving a follow-up endoscopy is for the most part independent of treatment status (ie, at random). This is supported by our findings that among those participants without a follow-up endoscopy, certain factors that may have affected the likelihood of receiving a follow-up endoscopy, such as age or stage of adenoma at baseline, did not differ by treatment status. It is also conceivable that occurrence of more-severe diseases during the follow-up could have affected the likelihood of receiving a follow-up endoscopy. In our trial, among the participants without a follow-up endoscopy, more participants in the placebo group had died or developed cancers than those in the FA group, but the number of deaths or cancers was quite small, and thus the number of undetected adenomas would be even smaller and should not have affected our results. In addition, baseline characteristics of participants included in the main analyses—ie, participants with an endoscopy during the follow-up period (n = 475)—were similar to those of all randomly assigned participants (n = 672). Because of all of the above discussed issues, we believe that that the lower rate of endoscopies in our trial could not have affected our findings considerably.
Fourth, we cannot exclude the possibility that our results may have been affected by participants who were taking multivitamins before random assignment; however, associations were similar when we examined associations separately by time between start of trial and first endoscopy (≤24 compared with >24 mo), which suggests that our results were not affected by participants who were taking multivitamins before being randomly assigned to their treatment group.
Fifth, this trial did not have a run-in period to assess compliance with pill-taking; however, comparison of folate concentrations measured at baseline and at midtrial indicated good compliance among participants with 2 blood samples. In addition, associations between FA supplementation and recurrent adenoma were similar after exclusion of participants who had informed us that they wished to stop taking pills or who had died during the study but for whom a report on endoscopy during the trial was obtained.
Finally, unlike the ukCAP and the AFPPS trial, participants in our more cost-efficient trial were not specifically required to undergo a colonoscopy before randomization. However, we conducted several sensitivity analyses and checked retrospectively whether randomized participants had received an endoscopy and were therefore adenoma free ≤2 y of randomization and found that associations between folic supplementation and recurrent adenoma did not differ appreciably when we restricted analysis to participants who, according to information obtained from medical records, had had an endoscopy up to 2 y before the start of the trial. Furthermore, the proportion of participants with recurrent adenoma in our trial was lower (28%) than that in the AFPPS trial (AFPPS trial: first follow-up interval, 43%; second follow-up interval, 40%) (5) and comparable to those in the ukCAP trial (26%) (6), which argues against a large number of prevalent baseline adenomas diluting our results.
In conclusion, our results do not support a protective effect of FA supplementation on the recurrence of colorectal adenomas. Contrary to findings from the AFPPS trial (5), but consistent with findings from the ukCAP trial (6), FA supplementation was not associated with higher risk of advanced adenomas or a higher number of noncolorectal cancers in our study. We found that FA supplementation may be beneficial among those with lower folate concentrations at baseline, especially those with low folate concentrations and high alcohol intake, which suggests the presence of a “folate-independent pathway” among those with a history of previous adenoma.
Acknowledgments
We thank all participants from the Health Professionals Follow-Up Study and the Nurses’ Health Study who participated in this trial. We also thank Lauren McLauglin, Mira Kaufman, Mike Atkinson, and Melissa Wei for their help in conducting this study.
The authors’ responsibilities were as follows—EG, DJH, and WCW: funding; EG, EAP, KW, WCW, DJH, and CSF: concept and design; EG, KW, EAP, JS, WCW, and CSF: data collection; KW: data analysis; BR and EG: statistical support; and all authors: manuscript writing and editing. None of the authors of this article had a conflict of interest to report.
REFERENCES
- 1.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr 2002;132:2350S–5S [DOI] [PubMed] [Google Scholar]
- 2.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen 2004;44:10–25 [DOI] [PubMed] [Google Scholar]
- 3.Jaszewski R, Misra S, Tobi M, et al. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World J Gastroenterol 2008;14:4492–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paspatis GA, Karamanolis DG. Folate supplementation and adenomatous colonic polyps. Dis Colon Rectum 1994;37:1340–1 [DOI] [PubMed] [Google Scholar]
- 5.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA 2007;297:2351–9 [DOI] [PubMed] [Google Scholar]
- 6.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 2008;134:29–38 [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Stampfer MJ, Colditz GA, et al. Folate, methionine, and alcohol intake and risk of colorectal adenoma. J Natl Cancer Inst 1993;85:875–84 [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987–91 [DOI] [PubMed] [Google Scholar]
- 9.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–8 [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Dietary fat and the risk of breast cancer. N Engl J Med 1987;316:22–8 [DOI] [PubMed] [Google Scholar]
- 11.Butterworth CE, Jr, Tamura T. Folic acid safety and toxicity: a brief review. Am J Clin Nutr 1989;50:353–8 [DOI] [PubMed] [Google Scholar]
- 12.Krumdieck CL. Folic Acid Brown ML, Present knowledge in nutrition. Washington, DC: International Life Sciences Institute, Nutrition Foundation, 1990:179–88 [Google Scholar]
- 13.Hathcock JN. Vitamins and minerals: efficacy and safety. Am J Clin Nutr 1997;66:427–37 [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration Food standards: amendment of standards of identity for enriched cereal grain products to require addition of folic acid. Fed Regist 1996;61:8781–97 [Google Scholar]
- 15.Giovannucci E, Stampfer MJ, Colditz GA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses' Health Study. Ann Intern Med 1998;129:517–24 [DOI] [PubMed] [Google Scholar]
- 16.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci USA 2007;104:19995–20000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich M, Brown CJ, Block G. The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr 2005;24:266–74 [DOI] [PubMed] [Google Scholar]