Abstract
Inflammation is associated with a number of chronic conditions, such as cancer and cardiovascular disease. Reducing inflammation may help prevent or treat these conditions. Diet has consistently been shown to modulate inflammation. To facilitate research into the inflammatory effect of diet on health in humans, we sought to develop and validate an Inflammatory Index designed to assess the inflammatory potential of individuals' diets. An Inflammatory Index was developed based on the results of an extensive literature search. Using data from a longitudinal observational study that carefully measured diet and the inflammatory marker, serum high-sensitivity (hs) C-reactive protein (CRP), in ∼600 adults for 1 y, we conducted analyses to test the effect of Inflammatory Index score on hs-CRP as a continuous and dichotomous (≤3 mg/L, >3 mg/L) indicator of inflammatory response, while controlling for important potential confounders. Results based on continuous measures of hs-CRP suggested that an increasing Inflammatory Index score (representing movement toward an antiinflammatory diet) was associated with a decrease in hs-CRP. Analyses using hs-CRP as a dichotomous variable showed that an antiinflammatory diet was associated with a decrease in the odds of an elevated hs-CRP (P = 0.049). The results are consistent with the ability of the Inflammatory Index to predict hs-CRP and provide additional evidence that diet plays a role in the regulation of inflammation, even after careful control of a wide variety of potential confounders.
Introduction
Inflammation has been associated with many chronic conditions, such as cancer, cardiovascular disease, obesity, and insulin resistance (1–4). There also is evidence that chronic inflammation may be associated with depression and may predispose individuals to dementia (5). Inflammation due to a response to injury, e.g. from cigarette smoking or hypertension, is involved in the steps of atherosclerosis that lead to plaque rupture and thrombosis (2,6). The inflammatory microenvironment includes production of cytokines and chemokines that also can lead to tumor initiation, growth, and invasion (7).
The acute-phase protein C-reactive protein (CRP)8 is produced in response to stimulation by interleukins (IL), such as IL-6 (8). Although used as a marker of inflammation in such conditions as rheumatoid arthritis for many decades, the more recent development of a high-sensitivity (hs)-CRP assay permitted the detection of inflammation at the vascular level (9). Many studies have shown CRP is associated with a number of cardiovascular disease endpoints (10). In addition, CRP and inflammatory cytokines, such as IL-6 and tumor necrosis factor-α (TNFα), are increased among obese individuals and positively correlated with BMI [weight (kg)/height (m)2] (3,4). Studies also have found that higher levels of IL-6 among obese individuals are associated with insulin resistance (3). Ridker et al. (11) found that each component of the metabolic syndrome (obesity, hypertriglyceridemia, low HDL-cholesterol, hypertension, abnormal glucose metabolism) is significantly associated with higher levels of hs-CRP.
Diet may play a central role in the regulation of chronic inflammation. The Western-type diet, which is high in red meat, high-fat dairy products, refined grains, and simple carbohydrates, has been associated with higher levels of CRP and IL-6 (12). On the other hand, the Mediterranean diet, which is high in whole grains, fish, fruit and green vegetables, and associated with moderate alcohol and olive oil intake and low intake of red meat and butter has been associated with lower levels of inflammation (13–17). Diets high in fruit and vegetable intake have been associated with lower levels of CRP (18–20). Specific nutrients such as (n-3) fatty acids (21–26), fiber (27–31), moderate alcohol intake (32–34), vitamin E (35–41), vitamin C (35,42–44), β-carotene (26,35,45,46), and magnesium (27,47–49) have also consistently been shown to be associated with lower levels of inflammation.
The overall goal of this project is to define and then validate an Inflammatory Index that assesses the inflammatory potential of the diet. Analyses are designed to determine how well the Inflammatory Index predicts interval changes in levels of hs-CRP (i.e. as a form of construct validation).
Methods
Development of the Inflammatory Index.
The goal in creating the Inflammatory Index was to provide a tool that could categorize individuals' diets on a continuum from maximally antiinflammatory to maximally proinflammatory. We created a score for each food and constituent thought to positively or negatively affect levels of inflammation. All peer-reviewed articles published in English between 1950 and 2007 that were identified as assessing the role of specific foods and constituents on specific inflammatory markers enumerated below were used to devise the score. A score was assigned to each food and constituent dependent on the findings from relevant peer-reviewed journal articles. Combined, this produced an overall score that summarized an individual's diet on the continuum from maximally anti inflammatory to maximally proinflammatory.
A literature search was performed to obtain articles that examined the association between inflammation and specific foods and constituents. We searched the National Library of Medicine database using PubMed and Ovid. We used 2 search engines to decrease the probability of missing relevant articles. Review articles were excluded from the search, because we needed to have the primary study results from each investigation. Furthermore, any article cited in reviews would be represented in the search. A list of terms was compiled to search for articles on inflammation. Due to the large number of articles on inflammation, the search was limited to 6 well-known inflammatory markers. The final list of inflammatory terms was: IL-1β, IL-4, IL-6, IL-10, TNFα, and CRP. Multiple variations for each of these terms were used in the search to decrease the chance of missing relevant articles. Similarly, variations in the name of foods and constituents were used to ensure that no appropriate articles were missed (i.e. full representation). Next, inflammatory terms were combined using the “or” option. Each food and constituent was individually combined with the list of inflammatory terms using the “and” option.
Based on the abstracts, articles were discarded for any of the following reasons: 1) did not examine the specific food/constituent and the inflammatory markers; 2) used inflammatory marker to stimulate other processes; 3) used a combination of foods/constituents as the exposure; 4) i.v. administration of constituent; 5) was published after the year 2007; 6) was a review paper; and 7) food-/constituent-specific exclusions (i.e. excluded studies looking at chronic alcohol exposure, alcohol abuse, or ethanol vapor).
All other full-text articles that were not discarded were retained for further review. Articles were again subject to the above exclusion criteria. All remaining articles were used in developing the Inflammatory Index (929 articles).
One of 3 possible values was assigned to each article based on the effect of the particular food or constituent on inflammation: −1 was assigned if the effects were proinflammatory (significantly increased IL-1β, IL-6, TNFα, or CRP or decreased IL-4 or IL-10); +1 if the effects were antiinflammatory (significantly decreased IL-1β, IL-6, TNFα, or CRP or increased IL-4 or IL-10); and 0 if the dietary variable produced no change in the inflammatory marker. In some instances, in a single study, constituents have been shown to decrease or increase both pro- and antiinflammatory markers. Also, there have been results that show an increase in one proinflammatory marker but a decrease in another. These are clear contradictions in the effect of a constituent on inflammation. To deal with this, we took the mean effect. There were cases, in a single study, where a constituent had no effect on a number of inflammatory markers but increased or decreased another. Scoring was based on the effect of the constituent on actual changes in inflammatory marker(s). For example, if a constituent did not affect levels of IL-6 or IL-1β, but significantly decreased CRP, the article was assigned +1.
Scoring the Inflammatory Index.
A number of steps were taken to determine how to score the Inflammatory Index. First, articles were weighted by study characteristics. Articles were weighted by the study type and design (Table 1). Second, using these weighted values, a score for each food and constituent was calculated. The following steps were used to calculate the score: 1) divided the weighted pro- and antiinflammatory articles by total weighted number of articles; and 2) subtracted the proinflammatory fraction from the antiinflammatory fraction (Fig. 1).
TABLE 1.
Study design weights
| Type of study | Study design | Value |
|---|---|---|
| Human | Experimental | 10 |
| Prospective cohort | 8 | |
| Case-control | 7 | |
| Cross-sectional | 6 | |
| Animal | Experimental | 5 |
| Cell culture |
Experimental |
3 |
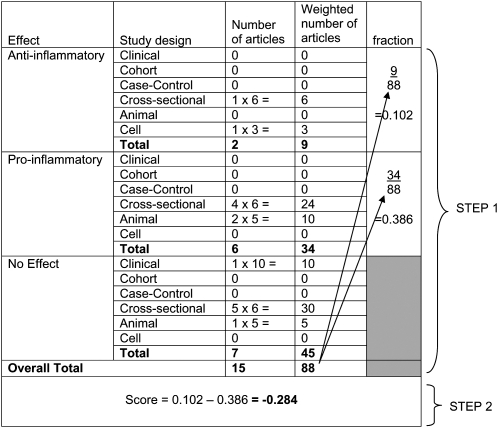
FIGURE 1 .
Example of how weighting was carried out for each food and constituent in the development of the Inflammatory Index. Saturated fat had a total of 15 articles, which resulted in 88 weighted. In step 1, articles were multiplied by assigned weights (Table 1). The total antiinflammatory and proinflammatory weight was divided by the total weight for saturated fat. In step 2, the proinflammatory fraction was subtracted from the antiinflammatory fraction.
Third, scores were adjusted for each food and constituent by the total weighted number of articles. An arbitrary cutpoint of 100 was chosen to indicate an optimally robust pool of literature. This number was used to adjust scores by weighting the number of articles. All foods and constituents with a weighted number of articles ≥ 100 were assigned the full value of the score. Foods and constituents with a weighted number of articles < 100 were adjusted as follows: 1) number of weighted articles was divided by 100; 2) the fraction was then multiplied by the score for that constituent, which resulted in the new adjusted score (i.e. for saturated fat: 88/100 = 0.88 × −0.284 = −0.25).
A list of all foods and constituents included in the Inflammatory Index along with the respective adjusted score are provided (Table 2). We did not include constituents or foods with total weighted articles of <10, which resulted in the exclusion of eugenol and 4 flavonoids, for which only 1 or 2 cell-culture studies existed.
TABLE 2.
Foods and constituents included in the Inflammatory Index
| Constituent | Adjusted score | Measure |
|---|---|---|
| Energy | −0.0549 | kJ/d |
| Energy* | −0.23 | kcal/d |
| Garlic | 0.27 | g/d |
| Ginger | 0.18 | g/d |
| Saffron | 0.18 | g/d |
| Turmeric | 0.774 | g/d |
| Tea | 0.552 | g/d |
| Caffeine | 0.035 | g/d |
| Wine | 0.48 | g/d |
| Beer | 0.2 | g/d |
| Liquor | 0.1 | g/d |
| Alcohol | 0.534 | g/d |
| Carbohydrate | −0.346 | g/d |
| Fiber | 0.52 | g/d |
| Fat | −0.323 | g/d |
| (n-3) Fatty acids | 0.384 | g/d x 10 |
| (n-6) Fatty acids | −0.016 | g/d x 10 |
| MUFA | −0.05 | g/d |
| Saturated fat | −0.25 | g/d |
| Protein | 0.05 | g/d |
| Cholesterol | −0.21 | mg/d |
| Vitamin A | 0.58 | μg/d ÷100 |
| Thiamin | 0.05 | mg/d |
| Riboflavin | 0.16 | mg/d |
| Niacin | 0.26 | mg/d |
| Vitamin B-6 | 0.286 | mg/d |
| Folic Acid | 0.214 | μg/d |
| Vitamin B-12 | −0.09 | μg/d |
| Vitamin C | 0.367 | mg/d |
| Vitamin D | 0.342 | μg/d |
| Vitamin E | 0.401 | mg/d |
| β-carotene | 0.725 | μg/d ÷ 100 |
| Magnesium | 0.905 | mg/d |
| Zinc | 0.316 | mg/d |
| Iron | 0.029 | mg/d |
| Selenium | 0.021 | mg/d |
| Quercetin | 0.49 | mg/d |
| Luteolin | 0.43 | mg/d |
| Genistein | 0.68 | mg/d |
| Daidzein | 0.17 | mg/d |
| Cyanidin | 0.13 | mg/d |
| Epicatechin |
0.12 |
mg/d |
The score was computed based on metric units (i.e., kcal/d).
Fourth, food- and constituent-specific scores were multiplied by the intake for each participant. Intake of vitamin A and β-carotene is recorded in the thousands of micrograms; thus, to equilibrate the range of intake to other micronutrients, they were divided by 100. Also, (n-3) and (n-6) fatty acids were multiplied by 10 because they are recorded in gram amounts and eaten in low quantities. Each food and constituent score product was then summed to create the overall Inflammatory Index score for each participant.
Fifth, the Inflammatory Index score was rescaled by dividing by 100 to aid in interpreting results of the statistical analyses.
Study design.
Details of the Seasonal Variation of Cholesterol Levels Study (SEASONS) are provided elsewhere (50,51). Briefly, SEASONS was a prospective observational study. A total of 641 healthy participants were followed for 1 y with measurements taken quarterly. Eligible participants were scheduled for an in-clinic appointment. At the first visit, previously completed questionnaires were obtained, anthropometric measurements were assessed, and a fasting blood sample was drawn. Follow-up appointments were scheduled every 3 mo for 1 y for a total of 5 appointments. There was a 6-wk window on both sides of each participant's quarterly appointment during which blood samples were obtained.
Considerable information was collected on each participant. Questionnaire-derived data included: demographics, psychosocial measures, social desirability and approval measures, seasonal patterns in mood and behavior, dietary information, and stress measures. Anthropometric measurements included: height, weight, waist circumference, hip circumference, and blood pressure. Serum hs-CRP was analyzed in the laboratory of Dr. Nader Rifai at Children's Hospital, Harvard Medical School, Boston, MA. Inter-assay and intra-assay CV for hs-CRP were in compliance with the U.S. CDC accepted ranges. The methodology was described previously (31). The Institutional Review Boards of the Fallon Healthcare System and the University of Massachusetts Medical School approved all participant recruitment and data collection procedures. Each participant signed an approved informed consent form before entering the study.
Dietary and physical activity assessment method.
In SEASONS, food and constituent intake data for each participant were obtained from the 24-h dietary recall interviews (24HR). Three randomly selected 24HR were collected at each quarter (including 2 weekdays and 1 weekend day) using the Nutrition Data System (NDS; DOS version 2.6, NDS 2.9) software. Information for all foods and constituents used in the Inflammatory Index was collected using this method except for flavonoid intake. The version of NDS that we used did not calculate intake of different flavonoids. Intakes of specific flavonoids were estimated from other sources, including the USDA's Database for the Flavonoid Content of Selected Foods (52) and the Iowa State University database on the isoflavone content of foods (53). Foods in these databases containing >5 mg/100 g of a specific flavonoid were included in the calculations for that specific flavonoid. The determination of foods containing the isoflavones genistein and daidzein was carried out similarly. To estimate flavonoid intake, content across selected foods was summed to determine overall intake per day. Flavonoids estimated using these methods were quercetin, epicatechin, cyanidin, luteolin, daidzein, and genistein. Physical activity data also were collected on the same sampling schedule during time allotted for the 24HR interviews according to methods we had developed and validated earlier (54,55).
Statistical analysis.
Summary statistics were used to describe the study population at baseline. Comparisons of baseline characteristics by gender were made using χ2 tests for categorical variables and 2-sample t tests for continuous variables. Statistical analyses were performed using linear mixed models (Proc MIXED in SAS) utilizing data from the Inflammatory Index, hs-CRP, and other time-varying covariates from each time point of study assessment. We used a compound symmetry covariance structure to account for the dependence of observations made on the same participants. The primary outcome variable for this analysis was the natural log of hs-CRP. Values of hs-CRP >10 mg/L were discarded, because this may be a result of acute inflammation (2). The primary independent variable was the interval-specific score obtained from the Inflammatory Index. The original statistical model and subsequent models controlled for various possible confounders and effect modifiers. Effect modification was assessed by stratified analyses and by including the interaction term in the model. Variables controlled in analyses were age, gender, race, BMI, smoking status, physical activity, energy intake, mean hours of sleep, highest level (year) of education attained, employment status, marital status, total cholesterol (TC), HDL-cholesterol, antiinflammatory medication use, light season, herbal supplement use, and infection status (a dichotomous variable indicating whether the participant had a self-reported infection during the study quarter). Race was dichotomized into White and other, because 90% of the study population was White. BMI was categorized into normal weight (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), and obese (≥30 kg/m2). Participant's considered to be underweight (<18.5 kg/m2) were excluded from analysis. Smoking status was dichotomized as yes/no. Dietary intake was measured using the 24HR method and reported as the mean daily consumption of nutrients and other exposures. Physical activity, measured as part of the 24HR interview process, was output as total metabolic equivalents (MET) over the quarter. Level of education was categorized into high school graduate or less, vocational/trade and some college, and college graduate or more. Employment status was categorized as full-time, part-time, or unemployed. Marital status was categorized into single, married, living with a partner, separated, divorced, or widowed. TC and HDL-cholesterol were left as continuous variables. Seasons were categorized using the “light season definition” centered at the equinoxes (winter: November 6 to February 4; spring: February 5 to May 6; summer: May 7 to August 5; and fall: August 6 to November 5). Participants who reported having arthritis were excluded from analysis. Also, observations missing hs-CRP, Inflammatory Index score, or any of the covariates used in the models were excluded from analysis.
Reports in the literature indicate that hs-CRP is not linear, but right-skewed (i.e. toward higher values) (2). Therefore, in addition to (natural) log transforming the data, we modeled the odds of elevated levels of hs-CRP (>3 mg/L) among individuals. Hs-CRP was dichotomized to ≤3 and >3 mg/L and used as the dependant variable in the model (2). To conduct this analysis we used the GLIMMIX procedure in SAS. As we did with Proc MIXED, we used the compound symmetry matrix to estimate the covariance structure and we fit the same covariates. All data analyses were performed using SAS software, version 9 (SAS Institute).
Results
A total of n = 519 participants had at least 1 clinic visit with an hs-CRP measurement and dietary intake collected using the 24HR method. Data from 1 participant were then excluded from analysis because of an hs-CRP value >10 mg/L (as this may be due to an underlying infection). Additionally, participants with arthritis (n = 2), a BMI <18.5 kg/m2 (n = 4), or missing any of the measurements for the covariates entered into the model (n = 18) were excluded. Thus, the final sample size for the analysis was 494. The number of repeat measurements available totaled 1670 (a mean of ∼3.4 complete repeats per participant).
Baseline characteristics of the study population are presented by gender (Tables 3 and 4). The majority of the study population was White, married, and working full time. The mean age was 48 y. Men were more likely than women to be overweight, married, working full time, and to have a higher level of education. A total of 68.9% of men and 71.7% of women reported having had an infection at some point during the study (data not shown).
TABLE 3.
Baseline characteristics of participants in the SEASONS (categorical variables)1
| Characteristic | Males, n = 264 | Females, n = 230 | P-value2 |
|---|---|---|---|
| Race | n (%) | 0.60 | |
| White (non-Hispanic) | 238 (90.2) | 204 (88.7) | |
| Other | 26 (9.8) | 26 (11.3) | |
| Current smoker | 0.47 | ||
| Yes | 49 (18.6) | 37 (16.1) | |
| No | 215 (81.4) | 193 (83.9) | |
| BMI | 0.01 | ||
| Normal weight (>18.5 to <25) | 82 (31.1) | 101 (43.9) | |
| Overweight (25 to <30) | 114 (43.2) | 80 (34.8) | |
| Obese (≥30) | 68 (25.7) | 49 (21.3) | |
| Marital status | 0.002 | ||
| Single | 26 (9.9) | 22 (9.6) | |
| Married | 209 (79.5) | 156 (67.8) | |
| Living with partner | 8 (3.0) | 12 (5.2) | |
| Separated | 3 (1.1) | 1 (0.4) | |
| Divorced | 13 (4.9) | 21 (9.1) | |
| Widowed | 4 (1.5) | 18 (7.8) | |
| Employment status | 0.0005 | ||
| Full time | 196 (74.2) | 134 (58.3) | |
| Part time | 29 (11.0) | 49 (21.3) | |
| Unemployed | 39 (14.8) | 47 (20.4) | |
| Job type | <0.0001 | ||
| Skill or craft | 27 (12.2) | 14 (8.1) | |
| Machine operator | 6 (2.7) | 0 (0.0) | |
| Manual labor | 16 (7.2) | 8 (4.6) | |
| Scientific technical work | 25 (11.3) | 8 (4.6) | |
| Service work | 20 (9.0) | 20 (11.6) | |
| Clinical, office, or sales | 16 (7.2) | 41 (23.7) | |
| Professional, managerial, or administrative work | 112 (50.4) | 82 (47.4) | |
| Education | <0.0001 | ||
| High school or less | 46 (17.4) | 84 (36.5) | |
| Vocational/trade or some college | 70 (26.5) | 47 (20.4) | |
| College or more |
148 (56.1) |
99 (43.1) |
|
Some of the categories do not sum to the total because of missing data.
P-value represents the difference between genders using χ2 tests.
TABLE 4.
Baseline characteristics of participants in the SEASONS (continuous variables)1
| Characteristic | Males, n = 264 | Females, n = 230 | P-value2 | P-value3 |
|---|---|---|---|---|
| Inflammatory index score | −1.0 ± 0.29 | −0.33 ± 0.20 | 0.06 | – |
| Age, y | 49.3 ± 0.76 | 48.4 ± 0.77 | 0.43 | – |
| Serum hs-CRP, mg/L | 2.4 ± 0.34 | 2.2 ± 0.48 | 0.74 | – |
| MET/d | 32.1 ± 0.47 | 29.1 ± 0.26 | <0.0001 | – |
| Total energy intake, kJ/d | 12070 ± 468.0 | 9008 ± 412.6 | <0.0001 | – |
| Total fat, g/d | 103.6 ± 4.30 | 75.8 ± 4.02 | <0.0001 | 0.49 |
| Saturated fat, g/d | 37.1 ± 1.61 | 26.8 ± 1.56 | <0.0001 | 0.40 |
| Protein, g/d | 113.5 ± 4.54 | 82.4 ± 3.56 | <0.0001 | 0.04 |
| Carbohydrates, g/d | 357.2 ± 15.14 | 284.4 ± 13.45 | 0.0004 | 0.004* |
| Fiber, g/d | 24.2 ± 1.39 | 19.6 ± 1.10 | 0.01 | 0.02* |
| Cholesterol, mg/d | 373.1 ± 16.87 | 251.3 ± 15.30 | <0.0001 | 0.01 |
| Vitamin A, μg/d | 1954.6 ± 216.1 | 1964.2 ± 105.8 | 0.11 | 0.35 |
| Folic acid, μg/d | 453.3 ± 26.40 | 400.0 ± 21.49 | 0.12 | 0.03* |
| Vitamin C, mg/d | 262.5 ± 29.21 | 203.6 ± 20.20 | 0.10 | 0.70 |
| Vitamin D, μg/d | 8.3 ± 0.64 | 7.4 ± 0.46 | 0.28 | 0.12 |
| Vitamin E, mg/d | 65.5 ± 7.72 | 49.0 ± 5.20 | 0.08 | 0.46 |
| β-Carotene, μg/d | 5538.8 ± 486.8 | 4932.5 ± 464.9 | 0.37 | 0.06 |
| Magnesium, mg/d | 427.4 ± 20.28 | 339.4 ± 15.26 | 0.0006 | 0.06 |
| Zinc, mg/d |
18.8 ± 1.02 |
14.5 ± 0.72 |
0.0006 |
0.49 |
Values are means ± SEM.
P-value represents the difference between genders using 2-sample t tests.
P-value representing the difference between genders after adjusting for energy intake. *Female energy adjusted intake is higher compared with males.
Energy intake was ∼3000 kJ higher among males compared with females. For most nutrients, males had a significantly higher intake compared with females. Generally higher nutrient intake among males was most likely attributable to a higher energy intake. Females had significantly higher intake of vitamin or mineral supplements (data not shown). No difference was found in herbal supplement use and very few participants consumed fish oil supplements (1.1% of men and 0.4% of women). Approximately 13% of the study population was using antiinflammatory drugs at baseline (this was controlled for during analysis). Across all time points, the Inflammatory Index score ranged from −20.9 to 24.7 (an ∼45-point range).
Analysis of hs-CRP as continuous.
The outcome variable, hs-CRP, was natural-log transformed, because it was not normally distributed (i.e. right-skewed) and the variance increased with increasing hs-CRP (heteroscedasticity). During the analysis of the Inflammatory Index score on log hs-CRP, certain potential effect modifiers were assessed. The interaction term for Inflammatory Index score and infection status during the study quarter was considered in the final model. However, stratification by infection status did not reveal a difference in the effect of Inflammatory Index score on log hs-CRP. Additionally, an interaction term with Inflammatory Index score and gender was fit and found not to be significant (data not shown). Therefore, no interaction term was entered into the final statistical model. An inverse but non-significant association was observed for Inflammatory Index score and log hs-CRP [regression coefficient (β) = −0.01] (Table 5). Age (years) was positively associated with log hs-CRP (β = 0.01; P = 0.0001). Participants who were nonsmokers had lower hs-CRP than active smokers (β = −0.18; P = 0.02). BMI was significantly positively associated with levels of hs-CRP. Compared with normal-weight participants, overweight and obese individuals had higher levels of hs-CRP (β = 0.38, P < 0.0001; β = 0.82, P < 0.0001, respectively). HDL-cholesterol was inversely related to hs-CRP (β = −0.009; P = 0.0009). Also, individuals without an infection during the study quarter had lower levels of hs-CRP (β = −0.15, P < 0.0001). No other variable entered into the model was significantly associated with hs-CRP.
TABLE 5.
Summary of linear regression analysis based on hs-CRP as a continuous outcome variable (log hs-CRP)1
| Variables | Estimate | SEE | P-value2 |
|---|---|---|---|
| Inflammatory Index score | −0.01 | 0.008 | 0.27 |
| Age, y | 0.01 | 0.004 | 0.0001 |
| HDL-cholesterol, mg/L | −0.009 | 0.003 | 0.0009 |
| Smoking status | |||
| Yes | 0 | 0 | 0 |
| No | −0.18 | 0.08 | 0.02 |
| BMI | |||
| Normal weight | 0 | 0 | 0 |
| Overweight | 0.38 | 0.07 | <0.0001 |
| Obese | 0.82 | 0.09 | <0.0001 |
| Infection status | |||
| Infection | 0 | 0 | 0 |
| No infection |
−0.15 |
0.04 |
<0.0001 |
Model was additionally adjusted for MET, gender, light season, race, education, energy intake, mean hours of sleep per day, TC, employment status, antiinflammatory medication use, and herbal supplement use.
P-value obtained from proc mixed.
Analysis of hs-CRP as dichotomous.
As recommended by the CDC and the AHA, we dichotomized hs-CRP at the level of 3 mg/L, considering measurements greater than this level at higher cardiovascular disease risk (2). A total of 301 observations (18% of the total 1670 observations) had an elevated hs-CRP, i.e. >3 mg/L. Results from this analysis revealed an inverse association between Inflammatory Index score and elevated hs-CRP (β = −0.06; P = 0.049) (Table 6). In addition, age (β = 0.02; P = 0.01), HDL-cholesterol (β = −0.02; P = 0.02), and absence of an infection (β = −0.35; P = 0.007) were associated with hs-CRP. BMI was also associated with hs-CRP. Overweight and obese individuals had higher odds of an elevated hs-CRP compared with normal-weight individuals (β = 0.59, P = 0.01; 1.56, P < 0.0001, respectively). No other variable entered into the model was significantly associated with hs-CRP. Similar to the analyses with log hs-CRP, we examined the association between Inflammatory Index score and the dichotomous hs-CRP with stratification by infection status. The effect of the Inflammatory Index score did not differ among those without an infection compared with those with an infection; therefore, we reported only the overall odds ratios here.
TABLE 6.
Summary of logistic regression analysis based on hs-CRP as a dichotomous variable (≤3, >3 mg/L)1
| Variables | Estimate | SEE | P-value2 |
|---|---|---|---|
| Inflammatory Index score | −0.06 | 0.03 | 0.049 |
| Age, y | 0.02 | 0.009 | 0.01 |
| HDL-cholesterol, mg/L | −0.02 | 0.008 | 0.02 |
| BMI | |||
| Normal weight | 0 | 0 | 0 |
| Overweight | 0.59 | 0.23 | 0.01 |
| Obese | 1.56 | 0.25 | <0.0001 |
| Infection status | |||
| Infection | 0 | 0 | 0 |
| No infection |
−0.35 |
0.13 |
0.007 |
Model was additionally adjusted for smoking status, MET, gender, light season, race, education, energy intake, mean hours of sleep per day, TC, employment status, antiinflammatory medication use, and herbal supplement use.
P-value obtained from proc glimmix.
A 5-point increase in score (∼10% of the total range) is associated with a reduced odds of an elevated hs-CRP (odds ratio, 0.76; 95% CI, 0.57, 0.998) (i.e. an ∼25% reduction). We also conducted additional analyses stratifying by gender and antiinflammatory medication use. Among females, the effect of Inflammatory Index score on hs-CRP category was slightly stronger (β = −0.07; P > 0.1) compared with males (β = −0.04; P > 0.1).
Discussion
This article describes the development and validation of the Inflammatory Index, a tool for assessing the inflammatory potential of the diet. We found evidence of an inverse association between the Inflammatory Index score and hs-CRP in this sample of 494 adult women and men, with a suggestion that increasing score (representing movement toward an antiinflammatory diet) was associated with a decrease in hs-CRP. An hs-CRP value of 3 mg/L is recommended as a cutoff point clinically, where values greater than this are considered to place individuals at high cardiovascular disease risk (2). Therefore, we performed additional analysis using hs-CRP as a dichotomous variable. We found that an antiinflammatory diet along the continuum of the Inflammatory Index was associated with a decrease in the odds of an elevated hs-CRP. When examining the effect of increasing Inflammatory Index score by 5 points (corresponding to roughly 10% of the full range of the score), we found significantly reduced odds for an elevated hs-CRP. These results indicate that an antiinflammatory diet may protect individuals from an inflammatory response characterized by elevated levels of hs-CRP and thus indirectly against the development of cancer, cardiovascular disease, and other inflammation-related chronic health conditions.
Although we observed significant associations when hs-CRP was examined as a dichotomous outcome, associations with the continuous hs-CRP variable were not significant. This may reflect the nonlinearity of the relationship between hs-CRP and diet. Similarly, in the paper by Fung et al. (56), there seemed to be a nonlinear association between dietary indices and CRP.
We found significant positive associations between age and hs-CRP, as observed previously (57). Also, BMI was significantly associated with hs-CRP in our study. Both overweight and, especially, obese participants had significantly higher levels of hs-CRP compared with normal-weight participants, a result consistent with existing literature, including those we reported previously (3,58).
It is noteworthy that other dietary indices exist and have been examined in association with inflammatory markers. However, to our knowledge, this is the first literature-based index to focus primarily on the inflammatory properties of the diet. The Inflammatory Index was developed based on our careful and systematic review of the literature. The literature review was conducted before any attempt was made to validate the measure in the SEASONS study database so as not to bias construction or validation of the index. Our results showing that an antiinflammatory diet (based on interval differences in the Inflammatory Index) is associated with a decrease in the odds of elevated hs-CRP are consistent with findings from studies using other dietary indices in the literature. Fung et al. (56) conducted a study where 5 dietary indices were assessed and their association with inflammatory markers was determined. Results from that study found that the Alternate Healthy Eating Index and the alternate Mediterranean Diet Index were significantly associated with CRP and IL-6 (56). However, given the role of inflammation in the pathophysiology of a number of chronic conditions, an index focused on the inflammatory potential of the diet may be better able to predict chronic disease outcomes. This needs to be tested in large epidemiologic studies having chronic disease-related endpoints.
There are strengths and limitations for both the Inflammatory Index and the data used for construct validation. Although the Inflammatory Index assesses diet as a whole, it was created using articles that examined the effect of single nutrients and other dietary constituents on inflammation. Examining nutrients in relation to disease outcomes can yield weaker relationships compared with food or dietary pattern intake (59,60). Another potential limitation of the Inflammatory Index is the dependence on certain arbitrary decisions made while determining the scoring algorithm. Waijers et al. (61) found that dietary indices do not predict disease or mortality any better than individual dietary factors and they attribute this to the arbitrary choices made during development of the indices. During development of the Inflammatory Index, certain weights and cutpoints were used to create a reasonable scoring algorithm. All decisions on the scoring algorithm were made a priori (i.e., before attempting to predict hs-CRP values). To check how robust the scoring algorithm was, additional analyses were conducted using cutpoints of 50 and 150. The point estimate for Inflammatory Index score was −0.04 using a cutpoint of 50 and −0.06 using a cutpoint of 150. The P-value for the Inflammatory Index score in these analyses were 0.06 and 0.046, respectively (hs-CRP as dichotomous). Another possible limitation of the Inflammatory Index is publication bias. Because the Inflammatory Index is dependent on the published literature, there is the possibility that inclusion of significant findings is more likely than null findings. Despite this, slightly under one-third of all associations contributing to the calculation of the Inflammatory Index score were null.
There also are strengths of the Inflammatory Index. The Inflammatory Index is based upon an extensive literature search incorporating cell culture, animal, and epidemiologic studies on the effect of diet on inflammation. As mentioned previously, the overall score is dependent on the whole diet, not just certain nutrients or foods. Inflammatory Index scoring is not dependent on population means or recommendations of intake; it is based on results published in the scientific literature. The Inflammatory Index is not limited to micronutrients and macronutrients but also incorporates commonly consumed components of the diet including flavonoids, spices, and tea.
One limitation of using the SEASONS study to validate the Inflammatory Index is its generalizability. ∼90% of the study population consisted of non-Hispanic Whites. Racial differences in hs-CRP have been noted (62). Thus, it would be beneficial to validate the Inflammatory Index in a more diverse population to assess possible differences in response between races. Finally, the version of NDS used for the 24HR interviews in the SEASONS study did not calculate trans fat intake. As a result, we were unable to incorporate trans fat into the calculation of the overall score.
There are a number of strengths in using the SEASONS study data. Dietary data were collected using the 24HR method. This type of dietary assessment method has been found to be the most accurate and efficient at measuring macronutrient and micronutrient intake, as well as being able to assess intake of foods not commonly found on food frequency questionnaires, such as spices, which may have a substantial effect on inflammation (63). Also, the sample size was large (494 participants contributing a total of 1,670 observations). Within the SEASONS, a wealth of information was collected at each time point. This allowed appropriate control for a number of other variables and decreased the possibility of residual confounding. It is important to note that the SEASONS is observational; it is remarkable to observe significant prediction of interval changes in hs-CRP by the Inflammatory Index score in the absence of an intervention.
The Inflammatory Index represents a new tool for assessing the inflammatory potential of the diet that can be applied to any population in which dietary data have been collected.
It also has the potential to be used for evaluating and guiding individuals in setting dietary goals to help decrease levels of inflammation and possibly reduce the risk of certain chronic conditions. The results from the validation of the Inflammatory Index in the SEASONS cohort provide additional evidence that diet plays a role in the regulation of inflammation, even after careful control of a wide variety of potential confounders.
These results provide support for one of the mechanisms through which dietary factors may decrease the risk of cancer, cardiovascular disease, and other inflammation-related conditions. Future research areas of interest include directly examining the association between the Inflammatory Index and these specific chronic disease outcomes.
Supplementary Material
Acknowledgments
P.P.C. helped design the Inflammatory Index, analyzed data, wrote the original draft of the paper, and had primary responsibility for final content. S.E.S. provided critical input in revising drafts of the paper. T.G.H. helped design statistical aspects of the SEASONS and the Inflammatory Index, analyzed data, helped write the paper, and provided statistical expertise. J. R. Hussey analyzed data and provided high-level statistical expertise and input in writing the paper. Y.M. and I.S.O. helped design the SEASONS and wrote drafts of the paper. J. R. Hébert helped design the SEASONS, guided design of the Inflammatory Index, analyzed data, and helped write the paper. All authors read and approved the final manuscript.
Supported by the South Carolina Statewide Cancer Prevention and Control Program and National Heart, Lung, and Blood Institute grant no. 1R01 HL073194-01 to support the SEASONS Study, I.S. Ockene, MD, P.I. and an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute to J. R. Hébert (K05 CA136975).
Author disclosures: P. P. Cavicchia, S. E. Steck, T. G. Hurley, J. R. Hussey. Y. Ma, I. S. Ockene, and J. R. Hébert, no conflicts of interest.
A list of published papers used in the development of the Inflammatory Index is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: CRP, C-reactive protein; 24HR, 24-hour dietary recall interview; hs, high-sensitivity; IL, interleukin; MET, total metabolic equivalent; NDS, Nutrition Data System; SEASONS, Seasonal Variation of Cholesterol Levels Study; TC, total cholesterol; TNFα, tumor necrosis factor-α.
References
- 1.Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Foundation Symposium. 2004;256:6–21; discussion 22–8, 49–52, 266–9. [PubMed] [Google Scholar]
- 2.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, Fadl YY, Fortmann SP, Hong Y, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 3.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338–42. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–200. [DOI] [PubMed] [Google Scholar]
- 5.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–56. [DOI] [PubMed] [Google Scholar]
- 6.Rosenson RS, Koenig W. Utility of inflammatory markers in the management of coronary artery disease. Am J Cardiol. 2003;92:i10–8. [DOI] [PubMed] [Google Scholar]
- 7.Jackson L, Evers BM. Chronic inflammation and pathogenesis of GI and pancreatic cancers. Cancer Treat Res. 2006;130:39–65. [DOI] [PubMed] [Google Scholar]
- 8.Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291:585–90. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–11. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–7. [DOI] [PubMed] [Google Scholar]
- 12.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–8. [DOI] [PubMed] [Google Scholar]
- 13.Dalziel K, Segal L, de Lorgeril M. A mediterranean diet is cost-effective in patients with previous myocardial infarction. J Nutr. 2006;136:1879–85. [DOI] [PubMed] [Google Scholar]
- 14.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44:152–8. [DOI] [PubMed] [Google Scholar]
- 15.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–6. [DOI] [PubMed] [Google Scholar]
- 16.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. [DOI] [PubMed] [Google Scholar]
- 17.Serrano-Martinez M, Palacios M, Martinez-Losa E, Lezaun R, Maravi C, Prado M, Martinez JA, Martinez-Gonzalez MA. A Mediterranean dietary style influences TNF-alpha and VCAM-1 coronary blood levels in unstable angina patients. Eur J Nutr. 2005;44:348–54. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Bermudez OI, Tucker KL. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. J Nutr. 2004;134:913–8. [DOI] [PubMed] [Google Scholar]
- 19.Watzl B, Kulling SE, Moseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. 2005;82:1052–8. [DOI] [PubMed] [Google Scholar]
- 20.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Fruit and vegetable intakes, C-reactive protein, and the metabolic syndrome. Am J Clin Nutr. 2006;84:1489–97. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–46. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–11. [DOI] [PubMed] [Google Scholar]
- 23.Niu K, Hozawa A, Kuriyama S, Ohmori-Matsuda K, Shimazu T, Nakaya N, Fujita K, Tsuji I, Nagatomi R. Dietary long-chain n-3 fatty acids of marine origin and serum C-reactive protein concentrations are associated in a population with a diet rich in marine products. Am J Clin Nutr. 2006;84:223–9. [DOI] [PubMed] [Google Scholar]
- 24.Zampelas A, Panagiotakos DB, Pitsavos C, Das UN, Chrysohoou C, Skoumas Y, Stefanadis C. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005;46:120–4. [DOI] [PubMed] [Google Scholar]
- 25.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–7. [DOI] [PubMed] [Google Scholar]
- 26.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR, Albanes D, et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483–7. [DOI] [PubMed] [Google Scholar]
- 27.Bo S, Durazzo M, Guidi S, Carello M, Sacerdote C, Silli B, Rosato R, Cassader M, Gentile L, et al. Dietary magnesium and fiber intakes and inflammatory and metabolic indicators in middle-aged subjects from a population-based cohort. Am J Clin Nutr. 2006;84:1062–9. [DOI] [PubMed] [Google Scholar]
- 28.King DE, Egan BM, Geesey ME. Relation of dietary fat and fiber to elevation of C-reactive protein. Am J Cardiol. 2003;92:1335–9. Erratum in: Am J Cardiol 2004;93:812. [DOI] [PubMed] [Google Scholar]
- 29.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134:1181–5. [DOI] [PubMed] [Google Scholar]
- 30.King DE, Egan BM, Woolson RF, Mainous AG III, Al-Solaiman Y, Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Arch Intern Med. 2007;167:502–6. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ III, Li W, Pagoto SL, Hafner AR, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avellone G, Di Garbo V, Campisi D, De Simone R, Raneli G, Scaglione R, Licata G. Effects of moderate Sicilian red wine consumption on inflammatory biomarkers of atherosclerosis. Eur J Clin Nutr. 2006;60:41–7. [DOI] [PubMed] [Google Scholar]
- 33.Sierksma A, van der Gaag MS, Kluft C, Hendriks HFJ. Moderate alcohol consumption reduces plasma C-reactive protein and fibrinogen levels; a randomized, diet-controlled intervention study. Eur J Clin Nutr. 2002;56:1130–6. [DOI] [PubMed] [Google Scholar]
- 34.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–7. [DOI] [PubMed] [Google Scholar]
- 35.van Herpen-Broekmans WM, Klopping-Ketelaars IA, Bots ML, Kluft C, Princen H, Hendriks HF, Tijburg LB, van Poppel G, Kardinaal AF. Serum carotenoids and vitamins in relation to markers of endothelial function and inflammation. Eur J Epidemiol. 2004;19:915–21. [DOI] [PubMed] [Google Scholar]
- 36.Bertran N, Camps J, Fernandez-Ballart J, Arija V, Ferre N, Tous M, Simo D, Murphy MM, Vilella E, et al. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J Lab Clin Med. 2005;145:41–6. [DOI] [PubMed] [Google Scholar]
- 37.Upritchard JE, Sutherland WH, Mann JI. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care. 2000;23:733–8. [DOI] [PubMed] [Google Scholar]
- 38.Devaraj S, Li D, Jialal I. The effects of alpha tocopherol supplementation on monocyte function. Decreased lipid oxidation, interleukin 1 beta secretion, and monocyte adhesion to endothelium. J Clin Invest. 1996;98:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Tits LJ, Demacker PN, de Graaf J, Hak-Lemmers HL, Stalenhoef AF. alpha-Tocopherol supplementation decreases production of superoxide and cytokines by leukocytes ex vivo in both normolipidemic and hypertriglyceridemic individuals. Am J Clin Nutr. 2000;71:458–64. [DOI] [PubMed] [Google Scholar]
- 40.Devaraj S, Jialal I. Alpha tocopherol supplementation decreases serum C-reactive protein and monocyte interleukin-6 levels in normal volunteers and type 2 diabetic patients. Free Radic Biol Med. 2000;29:790–2. [DOI] [PubMed] [Google Scholar]
- 41.Murphy RT, Foley JB, Tome MT, Mulvihill NT, Murphy A, McCarroll N, Crean P, Walsh MJ. Vitamin E modulation of C-reactive protein in smokers with acute coronary syndromes. Free Radic Biol Med. 2004;36:959–65. [DOI] [PubMed] [Google Scholar]
- 42.Chien CT, Chang WT, Chen HW, Wang TD, Liou SY, Chen TJ, Chang YL, Lee YT, Hsu SM. Ascorbate supplement reduces oxidative stress in dyslipidemic patients undergoing apheresis. Arterioscler Thromb Vasc Biol. 2004;24:1111–7. [DOI] [PubMed] [Google Scholar]
- 43.Korantzopoulos P, Kolettis TM, Kountouris E, Dimitroula V, Karanikis P, Pappa E, Siogas K, Goudevenos JA. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol. 2005;102:321–6. [DOI] [PubMed] [Google Scholar]
- 44.Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr. 2006;83:567–74, quiz 726–7. [DOI] [PubMed] [Google Scholar]
- 45.Erlinger TP, Guallar E, Miller ER III, Stolzenberg-Solomon R, Appel LJ. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch Intern Med. 2001;161:1903–8. [DOI] [PubMed] [Google Scholar]
- 46.Kritchevsky SB, Bush AJ, Pahor M, Gross MD. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol. 2000;152:1065–71. [DOI] [PubMed] [Google Scholar]
- 47.King DE, Mainous AG III, Geesey ME, Woolson RF. Dietary magnesium and C-reactive protein levels. J Am Coll Nutr. 2005;24:166–71. [DOI] [PubMed] [Google Scholar]
- 48.Song Y, Li TY, van Dam RM, Manson JE, Hu FB. Magnesium intake and plasma concentrations of markers of systemic inflammation and endothelial dysfunction in women. Am J Clin Nutr. 2007;85:1068–74. [DOI] [PubMed] [Google Scholar]
- 49.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:1438–44. [DOI] [PubMed] [Google Scholar]
- 50.Merriam PA, Ockene IS, Hebert JR, Rosal MC, Matthews CE. Seasonal variation of blood cholesterol levels: study methodology. J Biol Rhythms. 1999;14:330–9. [DOI] [PubMed] [Google Scholar]
- 51.Ockene IS, Chiriboga DE, Stanek EJ III, Harmatz MG, Nicolosi R, Saperia G, Well AD, Freedson P, Merriam PA, et al. Seasonal variation in serum cholesterol levels: treatment implications and possible mechanisms. Arch Intern Med. 2004;164:863–70. [DOI] [PubMed] [Google Scholar]
- 52.USDA, Agricultural Research Service. 2003. USDA Database for the Flavonoid Content of Selected Foods. Nutrient Data Laboratory [cited 2008 Jun 11]. Available from: http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/flav.html.
- 53.USDA, Agricultural Research Service. 2007. USDA-Iowa State University Database on the Isoflavone Content of Foods. Release 1.4–2007. Nutrient Data Laboratory [cited 2008 Jun 11]. Available from: http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html.
- 54.Matthews CE, Freedson PS, Hebert JR, Stanek EJ III, Merriam PA, Ockene IS. Comparing physical activity assessment methods in the Seasonal Variation of Blood Cholesterol Study. Med Sci Sports Exerc. 2000;32:976–84. [DOI] [PubMed] [Google Scholar]
- 55.Matthews CE, Hebert JR, Freedson PS, Stanek EJ III, Merriam PA, Ebbeling CB, Ockene IS. Sources of variance in daily physical activity levels in the seasonal variation of blood cholesterol study. Am J Epidemiol. 2001;153:987–95. [DOI] [PubMed] [Google Scholar]
- 56.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 57.Khor LL, Muhlestein JB, Carlquist JF, Horne BD, Bair TL, Maycock CA, Anderson JL. Sex- and age-related differences in the prognostic value of C-reactive protein in patients with angiographic coronary artery disease. Am J Med. 2004;117:657–64. [DOI] [PubMed] [Google Scholar]
- 58.Ockene IS, Matthews CE, Rifai N, Ridker PM, Reed G, Stanek E. Variability and classification accuracy of serial high-sensitivity C-reactive protein measurements in healthy adults. Clin Chem. 2001;47:444–50. [PubMed] [Google Scholar]
- 59.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 60.Mertz W. Foods and nutrients. J Am Diet Assoc. 1984;84:769–70. [PubMed] [Google Scholar]
- 61.Waijers PM, Feskens EJ, Ocke MC. A critical review of predefined diet quality scores. Br J Nutr. 2007;97:219–31. [DOI] [PubMed] [Google Scholar]
- 62.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH Jr, Grundy SM, de Lemos JA. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46:464–9. [DOI] [PubMed] [Google Scholar]
- 63.Hebert JR, Hurley TG, Chiriboga DE, Barone J. A comparison of selected nutrient intakes derived from three diet assessment methods used in a low-fat maintenance trial. Public Health Nutr. 1998;1:207–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.