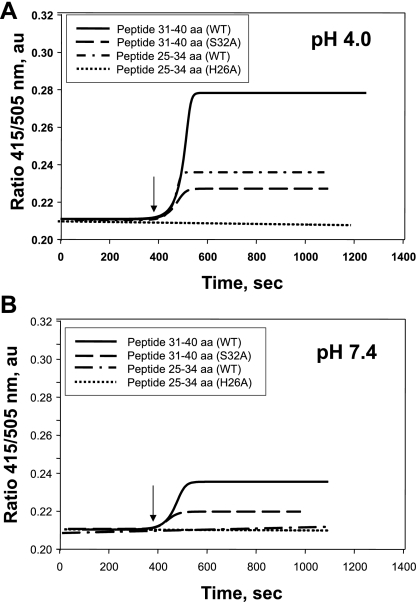
Fig. 5.
Binding of Prdx6 25–34 aa and 31–40 aa peptides to N-(5-dimethylaminonaphthalene-1-sulfonyl)-sn-glycero-3-phosphatidylethanolamine (N-DNS-PE) unilamellar liposomes. Liposomes (100 μM total phospholipid) were suspended in 40 mM sodium acetate buffer (pH 4.0) or 40 mM potassium phosphate buffer (pH 7.4). Binding was determined by measurement of the ratio of fluorescence emission at 415 nm to fluorescence emission at 505 nm. Binding curves of wild-type (WT) peptides and S32A and H26A mutants (100 μM) to N-DNS-PE liposomes at pH 4.0 and pH 7.4 are shown by a sigmoidal curve fit. Binding is shown in real time by detection of the DNS fluorescence ratio (415/505 nm). Arrow indicates addition of peptides to the liposome mixture.