Table 2.
One-pot synthesis of 2,3-disubstituted thiophenes from 2-TBS-3-formylthiophene 2b.
| Entry | R-M | E | Product(yield) | Entry | R-M | E | Product(yield) |
|---|---|---|---|---|---|---|---|
| 1 | n-BuLi | i-Pr-CHO | 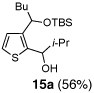 |
5 | Ph-Li | Ph-CHO | 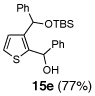 |
| 2 | n-BuLi | Ph-CHO | 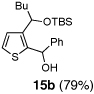 |
6 | Ph-CHO | 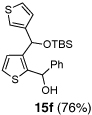 |
|
| 3 | n-BuLi | (Ph)2CO | 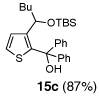 |
7 | i-Pr-CHO | 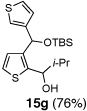 |
|
| 4 | Ph-Li | MeI | 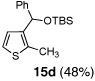 |
8 | n-BuLi | MeI | 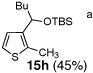 |
Condition: Step 1: 2b (1.0 eq.), Nucleophile (1.1 eq.), THF, −78 °C, 30 min;
Step 2: DMPU/THF = (1/1), Electrophile (1.5 eq.), −78 °C to RT, 3 h
We also obtained O-methylation product (15 i) in 41%
