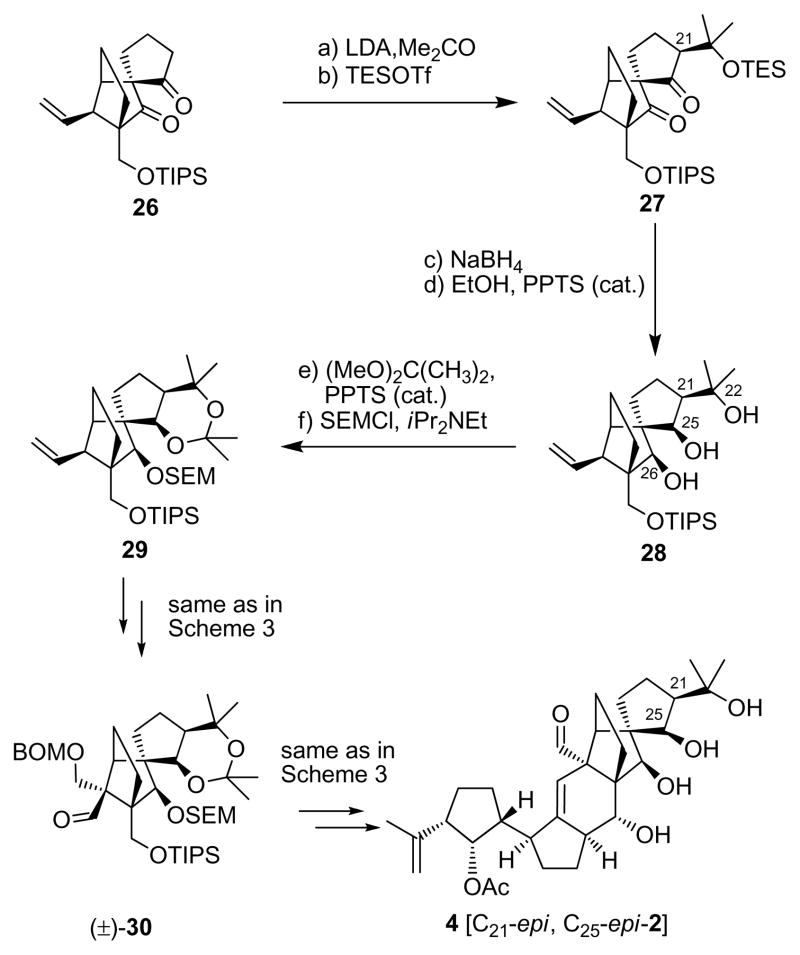
Scheme 4.
Synthesis of aldehyde (±)-30 and vannusal B structure 4. Reagents and conditions: (a) LDA [generated from iPr2NH (5.0 equiv), nBuLi (2.5 M in haxanes, 5.0 equiv)], THF, −78→−40 °C; then acetone (20 equiv), −40→25 °C, 1 h, (3:1 dr); (b) TESOTf (2.0 equiv), 2,6-lut. (5.0 equiv), −78→−40 °C, 1 h, 66 % for two steps; (c) NaBH4 (20 equiv), THF:MeOH (1:1), −10→25 °C, 4 h; (d) EtOH, PPTS (0.10 equiv), 25 °C, 2 h, 91 % for two steps; (e) (MeO)2C(Me)2:DMF (1:1), PPTS (1.0 equiv), 25 °C, 48 h, 100 %; (f) SEMCl (5.0 equiv), iPr2NEt (15 equiv), nBu4NI (1.0 equiv), CH2Cl2, 50 °C, 24 h, 97 %;