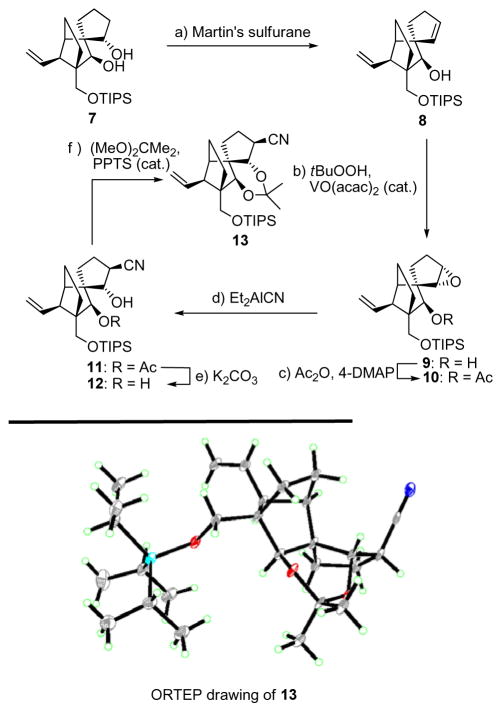
Scheme 1.
Construction of nitrile 12 (top) and X-ray derived ORTEP drawing of 13 (bottom). Reagents and conditions: (a) Martin’s sulfurane (1.1 equiv), Et3N (10 equiv), CH2Cl2, 25 °C, 5 h, 87 %; (b) tBuOOH (3.0 equiv), VO(acac)2 (0.2 equiv), PhH, 25 °C, 6 h, 90 %; (c) Ac2O (10 equiv), Et3N (30 equiv), 4-DMAP (1.0 equiv), CH2Cl2, 4 h, 25 °C, 90 %; (d) Et2AlCN (10 equiv), PhMe, −78→−20 °C, 19 h, 81 %; (e) K2CO3 (1.0 equiv), MeOH, 25 °C, 2 h, 100 %; (f) DMF/2,2-dimethoxypropane (1:1), PPTS (1.0 equiv), 24 h, 89 %.