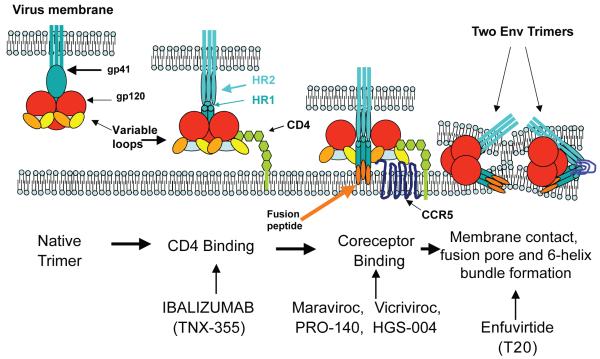
Figure 1. Therapeutic opportunities for inhibition of HIV-1 entry.
HIV-1 entry is mediated by the viral Env protein, which comprises the glycoproteins gp120 and gp41 arranged in trimeric spikes on the viral surface. Entry encompasses three steps: CD4 binding, coreceptor binding and fusion. The viral gp120 first binds to CD4, causing a repositioning of the variable loops V1/V2 and V3 and thereby exposing the bridging sheet and forming a coreceptor binding site. Upon coreceptor binding, conformational changes in gp120 and gp41 lead to the insertion of gp41 fusion peptide into the cell membrane. Subsequent conformational changes result in the formation of a six-helix bundle, with the HR2 domains folding back and packing into grooves on the outside of the triple-stranded HR1 domains. This brings the fusion peptide and transmembrane region of gp41 in close proximity, forming a fusion pore that allows transfer of the viral core into the cell. Each step on HIV-1 entry can be targeted by inhibitors currently approved or in clinical development. CD4 binding is targeted by ibalizumab (formerly TNX-355), a humanized monoclonal antibody that binds to CD4. Coreceptor binding is blocked by small-molecule CCR5 antagonists (maraviroc and vicriviroc) and by CCR5 antibodies (PRO-140 and HGS-004). Finally, the formation of a six-helix bundle, and thereby fusion, is prevented by enfuvirtide.145