Figure 4. IgG2 Dependence of Glycan Recognition by IVIg.
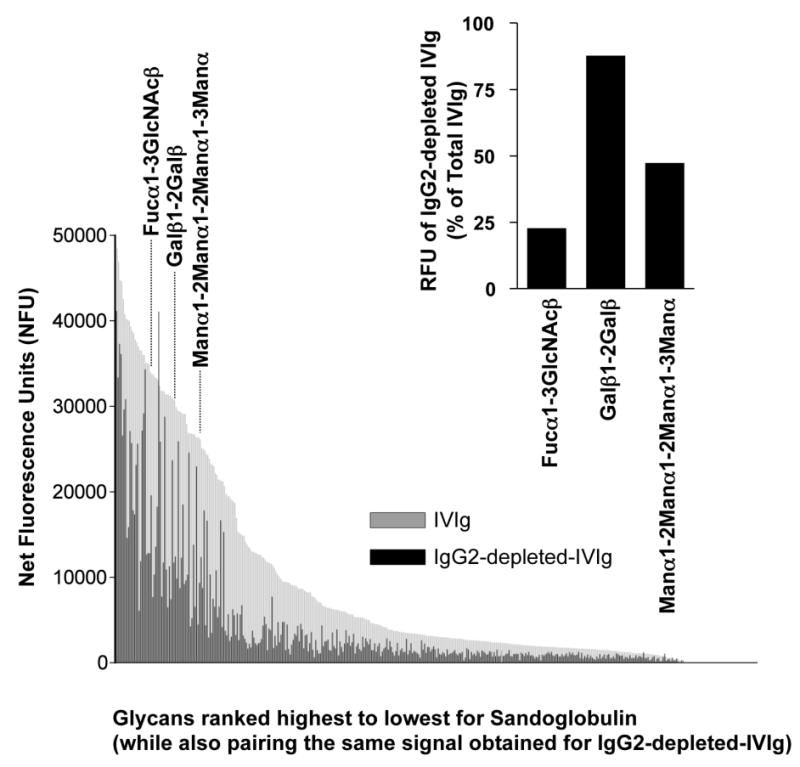
Glycans bound by IgG antibodies in the original and IgG2 depeted Sandoglobulin are ranked from highest to lowest binding. Signal intensities for IVIg binding to the 377 glycans were expressed as net fluorescence units after subtraction of the IgG control mix signal for each respective glycan. Depending on the carbohydrate structure, IgG2-depletion had different effects on glycan recognition by IVIg. For instance, binding to the three bacterial glycans Fucα1-3GlcNAcβ, Galβ1-2Galβ and Manα1-2Manα1-2Manα1-3Manα were largely IgG2-dependent, independent or mixed, respectively (see inset).
