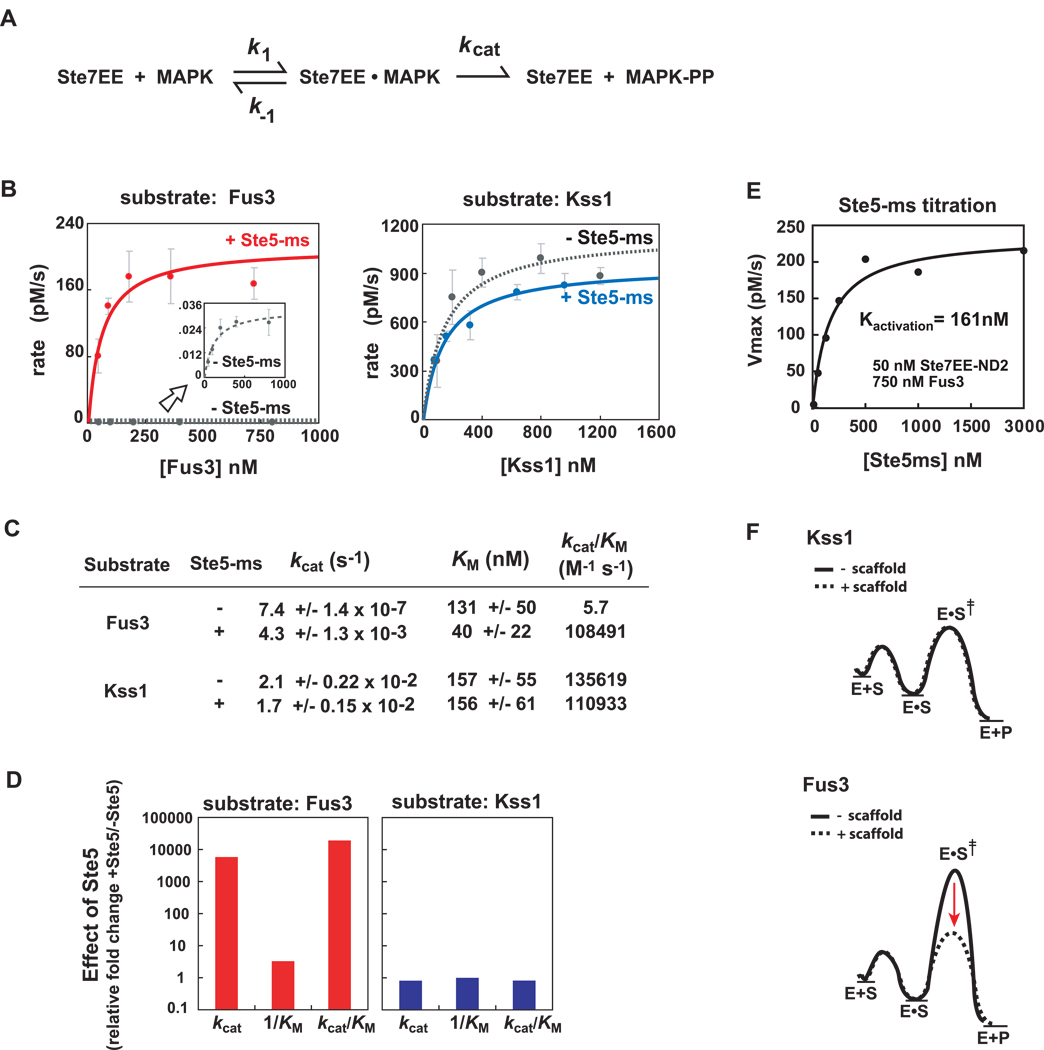
Figure 4. Ste5-ms domain selectively improves kcat (not KM) for the substrate, Fus3.
(A) Simple kinetic scheme for Ste7➔MAPK phosphorylation. Ste7EE enzyme converts substrate (MAPK) into doubly-phosphorylated product (MAPK-pp). Fus3 and Kss1 phosphorylation by Ste7EE was quantified using in vitro western blots with an anti-phospho p44/42 MAPK antibody (See Supp Fig. 1A–D and Supp Fig. 4). (B) Michaelis-Menten plots show Fus3 phosphorylation requires Ste5-ms, Kss1 phosphorylation does not. Ste7EE-ND2 (which contains only one MAPK docking motif, KD ~ 100nM), and Fus3-K42R (which is catalytically dead) were used to simplify the analyses. Kinase reactions contain 50nM Ste7EE-ND2, and a saturating concentration (1000nM, where appropriate) of Ste5-ms (see panel 4E). Fus3 activation by Ste7EE-ND2, in the absence Ste5-ms, is very slow but can be measured (inset graph). (C) Ste5-ms enhances the kcat of Ste7➔Fus3 phosphorylation by ~ 5000-fold, with negligible effect on KM. Ste5-ms has little or no effect on the kcat or KM of Kss1 phosphorylation. Overall specificity (kcat /KM) of Ste7 for Fus3 and Kss1 is comparable (~105 M−1 s−1). (D) Effect of Ste5 on Ste7➔MAPK phosphorylation reaction parameters, plotted as the fold-change in kcat, 1/KM, and kcatK/M for Fus3 and Kss1 activation by Ste7EE-ND2. Major effect of Ste5 is enhancement of the kcat for Fus3 phosphorylation. (E) Determination of the concentration of Ste5-ms required to drive Ste7➔Fus3 phosphorylation. 50nM Ste7EE was used along with a saturating amount of Fus3, (750nM, based on Fig. 4B). Rate of Fus3 activation reaches half-maximum at ~ 161nM Ste5-ms (+/− 60nM), which we infer is an apparent dissociation constant for the Ste7/Ste5-ms interaction. 1000 nM Ste5-ms, used in experiments described in panel 4B, represents a saturating concentration. (F) Reaction free energy diagram illustrating how Ste5-ms selectively lowers the energy of the transition state for Fus3 phosphorylation (dotted line).