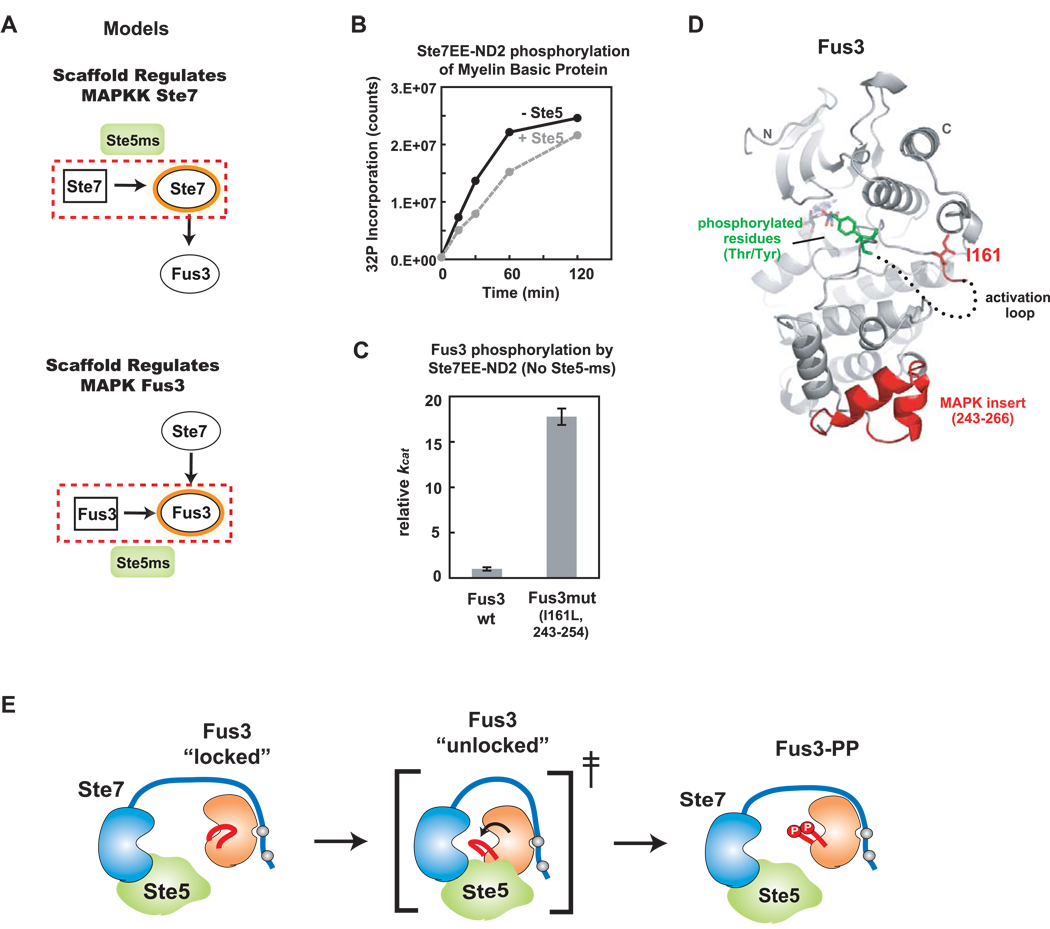
Figure 6. Ste5-ms catalytically unlocks Fus3 for phosphorylation by Ste7.
(A) Two potential models for how Ste5-ms enhances Ste7➔Fus3 phosphorylation. One model proposes that Ste5-ms primarily acts on Ste7; Ste7 is a poor enzyme that requires Ste5-ms binding to increase its activity (top). An alterative model hypothesizes that Ste5-ms acts primarily on Fus3 - converting it from a poor substrate to a good one (bottom). (B) Ste5-ms has no effect on overall catalytic activity of Ste7EE as tested against the general kinase substrate, Myelin Basic Protein (MBP) using a 32P kinase assay. (C) To identify elements in Fus3 that make it a poor substrate compared to Kss1, we made mutations in Fus3 that make it more similar in sequence to Kss1 (Supp. Fig. 8A,B). These mutants were tested for their ability to be phosphorylated by Ste7EE in the absence of Ste5 (Supp. Fig 8C–D). A combined mutation of I161L with replacement of the 243–254 ‘MAPK insertion loop’ (with the same region from Kss1) created a Fus3 mutant with a 20-fold increase in kcat compared to wild-type (reaction contains 50nM Ste7EE-ND2, 750nM Fus3 variant, no scaffold). (D) Crystal structure of Fus3 (Remenyi et al., 2005), showing positions of critical mutations in red (I161L, and MAPK insert 243–254). The activation loop (shown as dotted line; not fully visible in the crystal structure) sits between these two regions. Residues that become phosphorylated (T180 and Y182) shown in green. (E) A model for Ste5-ms action: Fus3’s activation loop normally adopts a “locked” conformation, but Ste5-ms interaction with Fus3 transiently (and only in the presence of Ste7) stabilizes a transition state in which Fus3’s activation loop is accessible to Ste7.