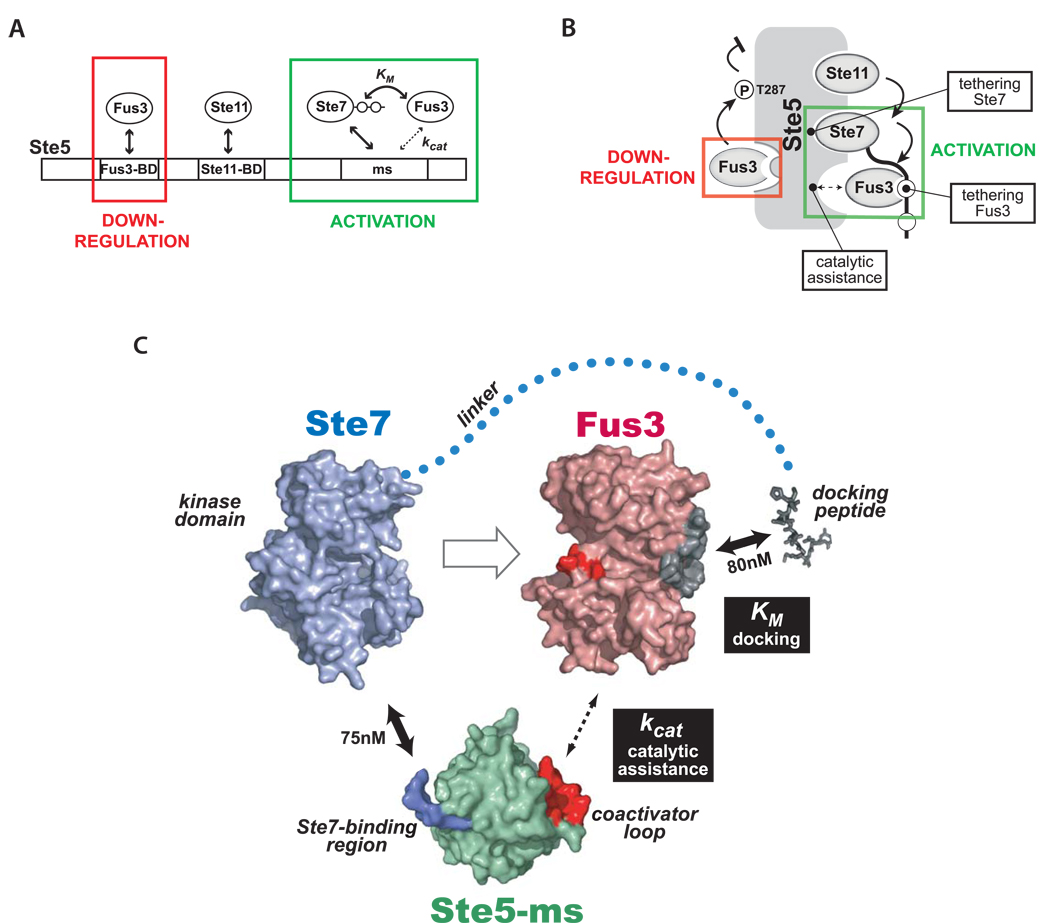
Figure 7. A new model for how the Ste5 scaffold controls information flow in the mating MAPK pathway.
(A) Ste5 has upregulatory (activating) and downregulatory interactions with Fus3. The strong, previously identified Fus3 binding site on Ste5 (Fus3-BD, KD = 1 µM) is not required for Ste7➔Fus3 phosphorylation, but rather is important for tuning down pathway output in vivo. Interactions that promote Fus3 phosphorylation involve the Ste5-ms domain (in cooperation with Ste7). (B) Cartoon summarizing various activities of Ste5. The Ste5-mediated complex has several critical tethering interactions (Ste5-Ste1 1, Ste5-Ste7, and Ste7-Fus3) essential for linear propagation of the mating pathway signal. In addition, Ste5-ms domain is an essential co-factor promoting catalysis of the Ste7➔Fus3 phosphorylation reaction. (C) Detailed model of minimal interactions in the mating scaffold complex required for Ste7➔Fus3 phosphorylation. Ste7 binds strongly to both Ste5-ms domain (via surface on Ste5-ms colored blue) and Fus3 (docking motifs on Ste7 bind to docking groove on Fus3 - colored gray), thereby tethering two proteins that normally interact only very weakly. Ste5-ms contains a coactivator loop (red surface) which promotes Fus3’s phosphorylation by Ste7. Fus3’s activation loop is colored red. Interaction affinities, where know, are indicated. Interactions that modulate kcat and KM of Fus3 phosphorylation by Ste7 are indicated by black boxes. Models for Fus3 (PDB code 2B9F) and Ste5-ms (this study) are derived from crystal structures. Ste7’s kinase domain was modeled from the structure of a homologous mammalian MAPKK (MKK7) using the threading program Phyre (Bennett-Lovsey et al., 2008).