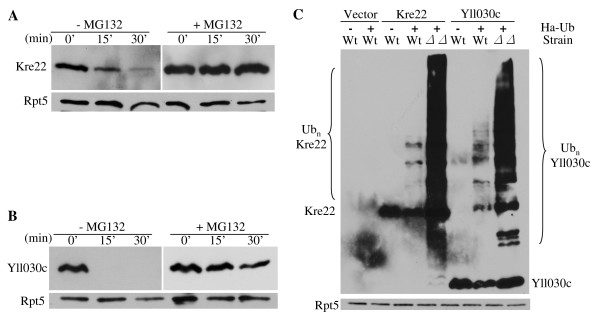
Figure 4.
Ubiquitin and the proteasome are involved in Kre22 and YLL030C degradation. (A-B) Kre22 and YLL030C are degraded by the proteasome. Wild-type yeast cells expressing Kre22 or YLL030C were treated with or without the proteasome inhibitor MG132. To facilitate the uptake of MG132 by wild-type yeast cells, we used L-proline as the nitrogen source in the growth medium and added a small amount of SDS (0.003%). (C) Kre22 and YLL030C are ubiquitylated. GST- and His6-tagged substrates were co-transformed with Ha-tagged Ub into wild-type or rad23 dsk2 mutant cells. Kre22 or YLL030C was precipitated with GST beads and analyzed by immunoblotting first with anti-Ha antibody and later anti-His6 antibody. Ubiquitylated and non-ubiquitylated Kre22 and YLL030C proteins are indicated on two sides of the upper panel. Rpt5 (bottom panel) is used as a loading control.