Abstract
Because of their physiological and life history characteristics, mammals exploit adaptive zones unavailable to ectothermic reptiles. Yet, they perform best in energy-rich environments because their high and constant growth rates and their sustained levels of resting metabolism require continuous resource supply. In resource-limited ecosystems such as islands, therefore, reptiles frequently displace mammals because their slow and flexible growth rates and low metabolic rates permit them to operate effectively with low energy flow. An apparent contradiction of this general principle is the long-term persistence of certain fossil large mammals on energy-poor Mediterranean islands. The purpose of the present study is to uncover the developmental and physiological strategies that allowed fossil large mammals to cope with the low levels of resource supply that characterize insular ecosystems. Long-bone histology of Myotragus, a Plio-Pleistocene bovid from the Balearic Islands, reveals lamellar-zonal tissue throughout the cortex, a trait exclusive to ectothermic reptiles. The bone microstructure indicates that Myotragus grew unlike any other mammal but similar to crocodiles at slow and flexible rates, ceased growth periodically, and attained somatic maturity extremely late by ≈12 years. This developmental pattern denotes that Myotragus, much like extant reptiles, synchronized its metabolic requirements with fluctuating resource levels. Our results suggest that developmental and physiological plasticity was crucial to the survival of this and, perhaps, other large mammals on resource-limited Mediterranean Islands, yet it eventually led to their extinction through a major predator, Homo sapiens.
Keywords: islands, artiodactyl, paleohistology, growth rate, metabolism
Energy availability is a key factor in the evolution of physiological and life history strategies of organisms. Therefore, much interest has recently been shown in the ecophysiological adaptations of vertebrates endemic to ecosystems with low energy flux (1). Ectotherms, although frequently thought of as primitive (2), are actually specialists in coping with low levels of available energy (3, 4). Ectotherm vertebrates have slow and flexible growth rates and a notable physiological plasticity, which allows a close matching of their energy requirements to prevailing resource conditions (3, 5, 6). Endotherms, instead, typically have high and steady growth rates and a constant thermometabolic regime, and they depend on high and continuous food intake to maintain their elevated metabolism (7). Therefore, in environments such as islands, where resource bases are narrow and resource availability is unpredictable (1), reptiles frequently replace mammals (8, 9).
Certain mammals, however, were dominant faunal elements on Mediterranean islands, where they persisted for long time periods, some of them over millions of years (10). This is particularly perplexing in the case of insular dwarf mammals such as elephants, deer, and hippos, which should be expected to have even higher resource requirements than small mammals because of the scaling of metabolic rate with body mass. Unsurprisingly, therefore, hypotheses aimed to explain the evolution of dwarfism and gigantism on islands (the Island Rule) (11) traditionally evoked resource availability as the driving force behind these, often dramatic, changes in body size (8, 9). More recently, however, several studies drew attention to the tight correlation between body size and life history traits, suggesting that not body size itself but fitness-related life history traits were the chief goal of selection on islands (12, 13). Thus, it has been argued that dwarfing is a corollary of selection for an increase in production rate in low-mortality environments (12–15) through an increase in growth rate (14) and a decrease in age at maturity (14, 15). This contrasts with a model that predicts shifts in adult body size in function of the magnitude of adaptive changes in growth rate and age at maturity in response to resource availability and extrinsic mortality (16). For environments such as islands, where resources are scarce and extrinsic mortality is low, this model predicts a decrease in adult body size through a decrease in growth rate, associated to an increase in age at maturity (16). Data that might provide empirical support for any of these essentially theoretical approaches, however, are scarce and come from observations on small extant vertebrates only (see ref. 16 for a more comprehensive review), because almost all large insular mammals went extinct following human settlement (1). The only way to reconstruct the physiological and life history strategies of dwarfed insular mammals, hence, is the study of their fossil remains. Myotragus, a dwarf bovid from the Plio-Pleistocene of Majorca (Balearic Islands, Spain), is particularly suitable for this purpose because it evolved under known selective pressures (chronically low resource levels and lack of predators) (10, 17) in a completely isolated ecosystem, conditions that closely resemble experiments on natural populations but at a timescale that only the fossil record can provide.
Physiological and life history strategies of fossil vertebrates are recorded in their hard tissues. Long-bone tissues of slow and flexibly growing ectotherms and fast and constantly growing endotherms differ substantially. Ectotherms are characterized by lamellar-zonal bone throughout the cortex. This bone is formed in a periodic manner whereby the deposition of lamellar (parallel-fibered) bone (18, 19) cyclically comes to a halt. These seasonal pauses in bone formation are recorded in the bone tissue as growth rings or lines of arrested growth (LAGs) (20–22). Endotherms are characterized by uninterrupted (azonal) fast growing fibrolamellar tissue throughout the cortex and a thin outer cortical layer (OCL) of slow growing lamellar bone deposited after attainment of somatic/sexual maturity (21, 23, 24). LAGs, if present, appear near the periosteum in the OCL (21, 23). An “intermediate” pattern, the fibrolamellar-zonal complex (25) composed of alternating zones of fibrolamellar tissue and LAGs, can be observed in extinct tetrapods only (dinosaurs and nonmammalian therapsids) (24, 25). Fossil evidence indicates that fast and uninterrupted growth has been acquired independently by birds and mammals (21). The capability to stop growth periodically is therefore considered to be a plesiomorphic trait reflecting an intermediate physiological condition (20) that has been lost in modern vertebrates (20, 24) or is simply a phylogenetic legacy (26).
Results
Ontogenetic Stages of Bone Tissue.
Our descriptions of bone tissues of Myotragus are based on the typological classification established by de Ricqlès (ref. 18; see also ref. 19). Thin sections from an ontogenetic series of 57 long bones of Myotragus reveal that the primary bone tissue consists of zonal bone throughout (Fig. 1A, C, E, and F; Fig. 2 B–E), comparable to that of crocodiles (compare Fig. 1 B and D). LAGs appear as simple (Fig. 1A, E, and F), double, or even triple rest lines (Fig. 2 B–E). They are spaced fairly homogeneously throughout the cortex (Fig. 2C). In older individuals, LAGs are closer spaced the more they approach the periosteal surface (Fig. 1A), indicating that growth rate decreased with age. At an early ontogenetic stage (Figs. 1F and 2B), fibrolamellar-zonal (23) tissue or lamellar bone with primary osteons (LPO) prevails, alternating with annuli of lamellar nonvascular bone (LNV) with flattened osteocytes and LAGs (Fig. 2 B and D). Vascularization is moderate with an essentially circumferential orientation of the channels. Early remodeling becomes manifest at the inner medullary surface (erosion of innermost primary tissue and deposition of inner circumferential layers, first Haversian systems) (Figs. 1F and 2B). The primary bone pattern of this early ontogenetic stage indicates a moderately rapid rate of bone deposition interrupted by low rates of bone deposition and growth arrest. It sharply contrasts with the early ontogenetic stage of other bovids (here Gazella borbonica, Fig. 1I), which is characterized by an azonal fibro-lamellar complex (FLC) throughout the cortex deposited during uninterrupted fast growth. At a later juvenile stage (Fig. 2 C and E), alternating LNV and LPO bone becomes predominant and vascularization decreases. Equidistant LAGs embedded in LNV tissue with flattened osteocytes (annuli) denote that growth slowed down and ceased periodically. Haversian systems become increasingly abundant throughout the inner half of the cortex. Older individuals (Fig. 1E) show very slow growing lamellar-zonal bone in which nonvascular annuli (LNV) and/or LAGs alternate with poorly vascularized zones (LNV/LPO) throughout cortex. This pattern of bone microstructure is frequent among wild alligators (27) (compare Fig. 1 B and D), but contrasts with the presence of fast growing fibrolamellar bone (FLC) throughout the cortex in other artiodactyls (here adult G. borbonica, Fig. 1H, and adult Cervus indet., Fig. 1G). None of the Myotragus specimens available for sectioning shows a distinct OCL that might indicate a rather abrupt onset of somatic maturity and/or sexual maturity as in other mammals. Instead, some of the specimens simply show an increasingly closer spacing of LAGs toward the outer cortex, a trait that characterizes crocodiles but not mammals (compare Fig. 1A with Eocene crocodile, Fig. 1B). Table 1 summarizes the main histological traits of Myotragus in comparison with the bone microstructure of crocodiles and large mammals.
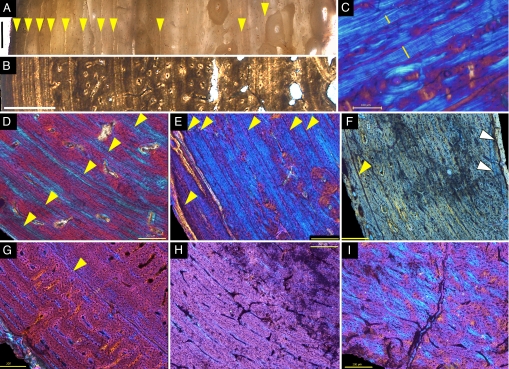
Fig. 1.
Micrographs of long bone tissues. (A) Myotragus balearicus (IPS 44929; entire section through cortical wall), adult distal tibia with completely fused epiphysis, 11 lines of arrested growth (LAGs), some Haversian systems. (B) Crocodile (IPS 4913, Eocene, Spain; entire section through cortical wall), adult femur. Observe the similarities with Myotragus (A) in the spacing of growth lines. (C) M. balearicus (IPS 44923c), subadult tibia, annuli (bars) interrupting FLC and LPO bone. (D) Crocodile gen. et sp. indet. (IPS 4930-h, Eocene, Spain), proximal femur with alternating lamellar annuli and fibrolamellar zones. (E) M. balearicus (IPS 44929), complete tibia with alternating LAGs, lamellar annuli, and fibrolamellar zones (elongated vascular channels, red). Note the resemblances with crocodile (D). (F) M. balearicus (IPS 26158-1), very tiny humerus of ≈4-cm length, FLC bone, one LAG (yellow arrowhead), two generations of endosteal bone (white arrowheads). (G) Cervid gen. et sp. indet. (IPS 3811-f, Pleistocene, Spain), adult distal tibia with completely fused epiphysis. Densely vascularized FLC tissue is shown with alternating formation of radial, concentric, and irregular oriented channels and one isolated LAG (arrowhead). Compare with the almost nonvascular zonal bone of Myotragus (E). (H) Gazella borbonica (IPS 26760-c, Pliocene, Spain), adult proximal femur with uninterrupted FLC bone. Compare with the zonal bone of adult Myotragus (E). (I) G. borbonica (IPS 26780, Pliocene, Spain) juvenile distal femur without epiphysis. Loosely formed azonal tissue of FLC type is shown with rounded osteocytes. Compare with the more compact and organized bone with flattened osteocytes and advanced remodeling of juvenile Myotragus (F). Periosteal surface is shown in all micrographs at lower left (left in A and B). (A and B) Transmitted light; (C–E and G–I) polarized light with 1λ filter; (F) circularly polarized light. (A and B) are composed of various micrographs. (Scale bars: A and D–I, 200 μm; B, 1,000 μm; C, 100 μm.)
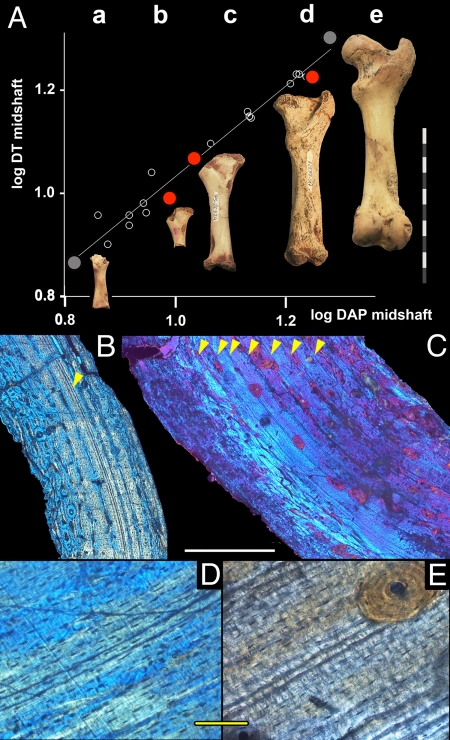
Fig. 2.
Growth series for three aged femora and micrographs of their sections. (A) Growth series of femora from the smallest juvenile (a, gray dot; mbcn7160) to a large adult individual (e, gray dot; mbcn7260) [logarithmic regression of anterior–posterior diameter (DAP) against transversal diameter (DT) at midshaft]. Sectioned specimens (red dots) provided ages of 2 (b, IPS 26444e), 3 (c, IPS 26324), and 8 (d, IPS 26321) years. Note the surprisingly small size at 2 and 3 years. (Scale bar, 10 cm.) (B) Section through cortical wall of IPS 26444e (femur in Ab) with a double LAG (arrowhead) in the central cortical wall, embedded in LNV annuli. Bone tissue is of FLC type close to the medullary cavity and of LPO type before and after the annuli. (C) Section through cortical wall of IPS 26321 (femur in Ad) with seven LAGs (arrowheads). Their regular distances and their presence only on the central cortex suggest that there might have been more LAGs that have been deleted by microbial attack (dark clouds) and remodeling; Haversian systems (red) scattered throughout inner cortex; erosion and endosteal bone surrounding medullary cavity. (D) Higher magnification of IPS 26444-e-1 (femur in Ab) showing the double LAG embedded in LNV tissue with flattened osteocytes. (E) Higher magnification of IPS 26321 (femur in Ad) showing multiple LAGs embedded in LNV tissue with flattened osteocytes. (Scale bars: B and C, 500 μm; D and E, 100 μm.) C is composed of multiple micrographs.
Table 1.
Main histological traits of crocodiles, large mammals, and Myotragus
| Histological traits | Crocodiles | Myotragus | Large mammals |
|---|---|---|---|
| Inner and central cortex | |||
| Primary bone | Zonal, LNV, LSV, and FLC | Zonal, LNV, LPO, and FLC | Azonal, FLC through LPO |
| Annuli | Present | Present | Absent |
| Resting lines (LAGs) | Cyclically throughout cortex | Cyclically throughout cortex | Rare, near periosteum |
| Outer cortical pattern in adults | Increasingly closer spacing of LAGs | Increasingly closer spacing of LAGs | OCL |
| Vascularization | Sparse to avascular | Sparse to avascular | Densely vascularized |
| Orientation of vascular channels | Mostly longitudinal | Longitudinal and concentric | Irregular, variable, increasingly organized with age |
| Remodeling | Little, in females extensive during egg-shell formation | Extensive | Extensive |
| Erosion and endosteal bone | Rare | From an early age onward | At subadult age |
| Haversian systems | Rare | Frequent | Extensive |
Bone microstructure of Myotragus is essentially similar to that of crocodiles in tissue pattern, periodicity of bone formation, transition to slower bone formation at skeletal/sexual maturity, and degree and pattern of vascularization. It resembles, however, other large mammals in pattern and rate of remodeling. LNV, lamellar nonvascular bone; LSV, lamellar bone with simple vascular canals; LPO, lamellar bone with primary osteons; FLC, fibrolamellar complex (18, 19). Trait description for crocodiles and large mammals is modified from refs. 21, 23, and 27.
Skeletochronology.
Skeletochronology is consistent with the slow and variable-rate growth pattern deduced from the long-bone tissue. The earliest ontogenetic stage available for sectioning is a very tiny and immature humerus without epiphyses (IPS 26158–1, length ≈4 cm; Fig. 1F). The tissue consists largely of FCL with longitudinal and circular osteons, although at the middle of the bone wall the tissue is more compact and of LPO type. Two clearly distinguishable generations of endosteal bone are deposited along the medullary cavity. At the periosteum, one LAG is observable followed by a thin annulus and, most peripherally, by FLC tissue, indicating that the individual resumed growth after the unfavorable season but died shortly after at the age of somewhat more than 1 year. A small proximal femur without epiphysis (IPS 26444e; Fig. 2 Ab, B, and D) shows FLC bone around the medullary cavity followed by LPO and LNV bone. Erosion is observable along the medullary cavity and some Haversian systems are scattered over the inner bone wall. At the middle of the bone wall there is a double LAG embedded in lamellar (LNV) annuli with flattened osteocytes, followed by alternating LPO/LNV bone. This tissue indicates that the individual recovered a faster growth rate after a period of slow growth and growth arrest. Age at death, hence, was at ≈2 years. A slightly larger immature femur (IPS 26324, Fig. 2Ac) that still lacks epiphyses, trochanter major, and trochanter minor shows little vascularized primary tissue of LPO type throughout. It presents two LAGs, large erosion cavities on the inner cortical (medullary) surface, endosteal bone, and more extensive Haversian remodeling, providing a minimum age of almost 3 years. We found a similar tissue pattern with two LAGs in a similar-sized humerus without epiphyses (IPS 26430). A minimum of six LAGs has been observed in a tibia of only two-thirds the size of a fully grown tibia in which the proximal epiphysis is not completely fused (IPS 44923-c), providing a minimum age of 7 years. Seven LAGs and, hence, a minimum age of close to 8 years, correspond to a juvenile femur of nearly adult size that still lacks both proximal and distal epiphyses and that shows an initial fusion of the trochanter major (IPS 26321; Fig. 2 Ad, C, and E). The primary bone largely consists of LNV tissue type; LAGs are mostly double or triple. Haversian systems invaded the inner cortical wall, and erosion and formation of endosteal bone along the medullary cavity are advanced. The presence of a minimum of 11 LAGs in fully grown individuals with epiphyses completely or almost completely fused (Fig. 1A, IPS 44929) denotes that Myotragus grew for at least 12 years before it attained skeletal/sexual maturity, more than sixfold the time of bovids of similar body mass (28) and even longer than large males of highly dimorphic Bison, which stop somatic growth at 7 years (29).
Discussion
The peculiar bone histology of Myotragus provides direct evidence of the developmental and growth strategy and indirect evidence regarding the physiology of this insular dwarf mammal. The presence of lamellar-zonal bone throughout the cortex indicates that Myotragus grew at slow and variable rates and ceased growth cyclically, which was associated with an important delay in the attainment of skeletal (sexual) maturity. Consistent with life history theory (30), the extended juvenile development of Myotragus was associated with an extended life span as indicated by the elevated number of very old individuals in the fossil assemblages (10). Our empirical finding, hence, does not support the prediction that life history traits of insular dwarfs accelerate to increase reproductive investment (12–15), but instead lends support to the model that predicts a shift in life history traits toward the slow end of the slow–fast continuum with a delay in age at maturity and an extended life span (16).
True zonal bone with growth marks deposited seasonally throughout ontogeny is a general ectotherm characteristic (20). In ectotherms, the bone matrix consists of slow growing lamellar bone (LNV, LSV, LPO). However, cyclically interrupted fast growing fibrolamellar bone (fibrolamellar-zonal complex, ref. 25) has been found in many dinosaur taxa, in basal birds (20, 31) and in nonmammalian therapsids (24), leading to an ongoing controversy over whether zonal bone indicates an intermediate physiological condition along the transition between poikilothermic ectothermy and homeothermic endothermy (20) or whether it merely represents the ghost of past physiologies (26). Inferences about the physiologies of these extinct vertebrate groups, however, remain conjectural because they do not have living equivalents. Our finding of true lamellar-zonal bone in a fossil representative of phylogenetically modern mammals, hence, may shed some light on the physiological correlates of zonal bone.
Ungulates, like other endotherms, are characterized by azonal fast growing bone tissue and a thin outer cortical layer that may contain several growth lines. Sporadically, a single, isolated LAG has been observed within the fast growing fibrolamellar bone of cervids (23, 26) (see also Fig. 1G). The occasional presence of LAGs in these large endothermic (nonhibernating and nonestivating) mammals led some (26) to conclude that such growth lines reflect phylogenetic legacy rather than a physiological response to environmental cycles or stresses. Nevertheless, evidence is accumulating that certain ungulates significantly reduce endogenous heat production to cope with energetically challenging situations (food shortage, harsh climatic conditions) (32). Thus, seasonal fluctuations in metabolic rate and in body temperature (heterothermy) have been described for ungulates with a winter nadir in northern species (32–34) and with a summer nadir in desert species (35, 36). Taking into account these recent advances in ungulate physiology, the zonal bone of Myotragus quite likely reflects seasonal fluctuations in metabolic rate and/or body temperature over an extended juvenile period in response to fluctuating resource conditions on the island.
Insular ecosystems are intrinsically resource limited (1, 8, 9, 16, 37) because their limited landmass can support only a limited number of primary producers, which in turn affects the energy flow at higher trophic levels. Therefore, energy-poor islands are depauperate in competitors and predators (8, 9). Under these conditions, the pivotal achievements of endothermy—(i) sustained aerobic capacities (7), (ii) an enhanced behavioral repertoire (7), (iii) high growth rates (38), and (iv) high reproductive rates (38)—are not only dispensable but the elevated metabolic rate to fuel these activities is also incompatible with the low insular resource bases. Therefore, insular endotherms should be expected to reduce these expensive traits. Indeed, among extant birds and mammals, small-island endemics have lower basal metabolic rates than their continental counterparts (8, 9, 39), whereas heterothermic small mammals such as dormice were dominant faunal elements in the fossil record of Mediterranean islands (10).
Majorca was such a resource-poor island, which is evidenced by nutrition-related malformations and other symptoms of starvation and undernourishment in fossil endemic populations (10), as well as by the low species diversity and the absence of predators (10). In agreement with the reasoning given above, Myotragus not only decreased aerobic capacities (low-gear locomotion) (40) and behavioral traits (reduction of brain and sense organs) (41) but also flexibly synchronized growth rates and metabolic needs to the prevailing resource conditions as do ectothermic reptiles. Our present study, hence, provides evidence that in energy-poor environments where reptiles usually replace mammals, selection for energy saving may be so imperious that mammals may revert to some ectotherm-like state that includes both physiological and developmental plasticity. Completely unexpected, this reversal is possible even in large mammals of phylogenetically modern groups such as bovids.
The reptile-like physiological and life history traits found in Myotragus were certainly crucial to their survival on a small island for the amazing period of 5.2 million years, more than twice the average persistence of continental species (42). Therefore, we expect similar physiological and life history traits to be present in other large insular mammals such as dwarf elephants, hippos, and deer. However, precisely because of these traits (very tiny and immature neonates, low growth rate, decreased aerobic capacities, and reduced behavioral traits), Myotragus did not survive the arrival of a major predator, Homo sapiens, some 3,000 years ago.
Materials and Methods
Materials.
Fossil material is as follows: Myotragus, Plio-Pleistocene of Majorca, Spain (Cova de Llenaire, Es Bufador, Son Apats, Cova de Moleta, Avenc de Nècora); crocodile (genus and species indet.), Eocene (Lutetian), La Boixedat (Huesca, Spain); G. borbonica, Middle Pliocene, Layna (Spain); cervid (genus and species indet.), Upper Pliocene, Vilarroya (Spain). Specimens labeled IPS are housed at the Institut Català de Paleontologia, Universitat Autònoma de Barcelona, Bellaterra, Spain. Specimens labeled mbcn are housed at the Museu Balear de Ciències Naturals, Sóller, Majorca (Spain).
To avoid irreversible damaging of valuable material, we preferred fragmented specimens for sectioning (except for IPS 44923-c, IPS 26158–1, IPS 26324, and IPS 26321). Slices were made at midshaft following standard procedures (21, 23) and examined under transmitted light and under polarized and circularly polarized light with a 1λ filter. Micrographs (Figs. 1 C–I and 2 B, D, and E) were taken on slices previously moistened with a drop of alcohol (98%) on their uncovered surface (a common procedure in petrography and crystallography). This procedure emphasizes the original tissue structure where this is affected by microbial attack or diagenetic processes, without damaging the fossil. Micrographs were taken with a polarization microscope (Leica DM 2500 P).
Age Assessment.
There is a general agreement that growth marks represent annual cycles (21, 22, 25). We estimated the age of individuals by counting cortical growth rings in histological sections of each specimen. Estimation of the number of lost or masked growth marks was not possible because removal of inner cortical bone starts at early ontogenetic stages. Therefore, estimated ages are minimum ages.
Acknowledgments.
We thank A. de Ricqlès, A. Chinsamy-Turan, and M. Sander for helpful comments on the micrographs; L. Demetrius for constructive discussions on the manuscript; two anonymous referees; L. Celià for assistance in the collections of the Catalan Institute of Paleontology; C. Constantino for access to the collections of the Museu Balear de Ciències Naturals (Sóller, Spain); and R. García and A. García for technical help. This work was supported by the Spanish Ministry of Science and Innovation (CGL2008–06204/BTE) and by the National Science Foundation (RHOI-Hominid-NSF-BCS-0321893).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.McNab B. The Physiological Ecology of Vertebrates: A View from Energetics. Ithaca, NY: Cornell Univ Press; 2002. [Google Scholar]
- 2.Cox CB. In: Looking at Animals Again. Arthur DR, editor. New York; San Francisco: Freeman; 1966. pp. 97–118. [Google Scholar]
- 3.Shine R. Life-history evolution in reptiles. Annu Rev Ecol Evol Syst. 2005;36:23–46. [Google Scholar]
- 4.Pough FH. In: Behavioral Energetics. Aspey WP, Lustick SI, editors. Columbus, OH: Ohio State Univ Press; 1980. pp. 141–189. [Google Scholar]
- 5.Lance VA. Alligator physiology and life history: The importance of temperature. Exp Gerontol. 2003;38:801–805. doi: 10.1016/s0531-5565(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 6.Rootes WL, Wright VL, Brown RW, Hess TJ. Growth rates of American alligators in estuarine and palustrine wetlands in Louisiana. Estuaries. 1991;14:489–494. [Google Scholar]
- 7.Bennett AF, Ruben JA. Endothermy and activity in vertebrates. Science. 1979;206:649–654. doi: 10.1126/science.493968. [DOI] [PubMed] [Google Scholar]
- 8.McNab B. Resource use and the survival of land and freshwater vertebrates on oceanic islands. Am Nat. 1994;144:643–660. [Google Scholar]
- 9.McNab BK. Minimizing energy expenditure facilitates vertebrate persistence on oceanic islands. Ecol Lett. 2002;5:693–704. [Google Scholar]
- 10.Alcover JA, Moyà-Solà S, Pons-Moyà J. Chimeras of the Past. Palma de Mallorca, Spain: Editorial Moll; 1981. (Translated from Catalan) [Google Scholar]
- 11.Van Valen L. Pattern and the balance of nature. Evol Theory. 1973;1:31–49. [Google Scholar]
- 12.Brown JH, Marquet PA, Taper ML. Evolution of body size: Consequences of an energetic definition of fitness. Am Nat. 1993;142:573–584. doi: 10.1086/285558. [DOI] [PubMed] [Google Scholar]
- 13.Brown JH, Sibly RM. Life-history evolution under production constraint. Proc Natl Acad Sci USA. 2006;103:17595–17599. doi: 10.1073/pnas.0608522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raia P, Barbera C, Conte M. The fast life of a dwarfed giant. Evol Ecol. 2003;17:293–312. [Google Scholar]
- 15.Raia P, Meiri S. The island rule in large mammals: Paleontology meets ecology. Evolution. 2006;60:1731–1742. [PubMed] [Google Scholar]
- 16.Palkovacs E. Explaining adaptive shifts in body size on islands: A life history approach. Oikos. 2003;103:37–44. [Google Scholar]
- 17.Sondaar PY. In: Major Patterns of Vertebrate Evolution. Hecht MK, Goody PC, Hecht BM, editors. Plenum Press; 1977. pp. 671–707. [Google Scholar]
- 18.Ricqlès A, de Meunier FJ, Castanet J, Francillon-Vieillot H. In: Bone Matrix and Bone Specific Products. Hall BK, editor. Vol 3. Boca Raton, FL: CRC Press; 1991. pp. 1–78. [Google Scholar]
- 19.de Margerie E, Cubo J, Castanet J. Bone typology and growth rate: Testing and quantifying ‘Amprino's rule’ in the mallard (Anas platyrhynchus) C R Biol. 2002;325:221–230. doi: 10.1016/s1631-0691(02)01429-4. [DOI] [PubMed] [Google Scholar]
- 20.Chinsamy A, Chiappe LM, Dodson P. Mesozoic avian bone microstructure: Physiological implications. Paleobiology. 1995;21:561–574. [Google Scholar]
- 21.Chinsamy A. The Microstructure of Dinosaur Bone: Deciphering Biology with Fine Scale Techniques. Baltimore: Johns Hopkins Univ Press; 2005. [Google Scholar]
- 22.Erickson GM. Assessing dinosaur growth patterns: A microscopic revolution. Trends Ecol Evol. 2005;20:677–684. doi: 10.1016/j.tree.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Klevezal GA. Recording Structures of Mammals: Determination of Age and Reconstruction of Life History. Rotterdam: AA Balkema; 1996. [Google Scholar]
- 24.Ray S, Botha J, Chinsamy A. Bone histology and growth patterns of some nonmammalian therapsids. J Vertebr Paleontol. 2004;24:634–648. [Google Scholar]
- 25.Castanet J. Time recording in bone microstructures of endothermic animals; functional relationships. C R Palevol. 2006;5:629–636. [Google Scholar]
- 26.Horner JR, de Ricqlès A, Padian K. Long bone histology of the hadrosaurid dinosaur Maiasaura peeblesorum: Growth dynamics and physiology based on an ontogenetic series of skeletal elements. J Vertebr Paleontol. 2000;20:115–129. [Google Scholar]
- 27.Tumarkin-Deratzian AR. Fibrolamellar bone in wild adult alligator mississippiensis. J Herpetol. 2007;41:341–345. [Google Scholar]
- 28.Magalhaes JP, Costa J, Toussaint O. HAGR: The Human Ageing Genomic Resources. Nucleic Acids Res. 2005;33(Database Issue):D537–D543. doi: 10.1093/nar/gki017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krasinska M, Krasinski ZA. Body mass and measurements of the European bison during postnatal development. Acta Theriol. 2002;47:85–106. [Google Scholar]
- 30.Stearns SC. The Evolution of Life Histories. New York: Oxford Univ Press; 1992. [Google Scholar]
- 31.Starck JM, Chinsamy A. Microstructure and developmental plasticity in birds and other dinosaurs. J Morphol. 2002;254:232–246. doi: 10.1002/jmor.10029. [DOI] [PubMed] [Google Scholar]
- 32.Arnold W, et al. Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus) Am J Physiol. 2004;286:R174–R181. doi: 10.1152/ajpregu.00593.2002. [DOI] [PubMed] [Google Scholar]
- 33.Arnold W, Ruf T, Kuntz R. Seasonal adjustment of energy budget in a large wild mammal, the Przewalski horse (Equus ferus przewalskii) II. Energy expenditure. J Exp Biol. 2006;209:4566–4573. doi: 10.1242/jeb.02536. [DOI] [PubMed] [Google Scholar]
- 34.Ringberg T. The Spitzbergen reindeer—A winter-dormant ungulate? Acta Physiol Scand. 1979;105:268–273. doi: 10.1111/j.1748-1716.1979.tb06341.x. [DOI] [PubMed] [Google Scholar]
- 35.Ostrowski S, Williams JB, Ismael K. Heterothermy and the water economy of free-living Arabian oryx (Oryx leucoryx) J Exp Biol. 2003;206:1471–1478. doi: 10.1242/jeb.00275. [DOI] [PubMed] [Google Scholar]
- 36.Ostrowski S, Williams JB. Heterothermy of free-living Arabian sand gazelles (Gazella subgutturosa marica) in a desert environment. J Exp Biol. 2006;209:1421–1429. doi: 10.1242/jeb.02151. [DOI] [PubMed] [Google Scholar]
- 37.Lomolino M. Body size evolution in insular vertebrates: Generality of the island rule. J Biogeogr. 2005;32:1683–1699. [Google Scholar]
- 38.McNab B. The energetics of reproduction in endotherms and its implication for their conservation. Int Comp Biol. 2006;46(6):1159–1168. doi: 10.1093/icb/icl016. [DOI] [PubMed] [Google Scholar]
- 39.McNab B, Bonaccorso FJ. The metabolism of New Guinean pteropodid bats. J Comp Physiol B. 2001;171:201–214. doi: 10.1007/s003600000163. [DOI] [PubMed] [Google Scholar]
- 40.Köhler M, Moyà-Solà S. Phalangeal adaptations in the insular fossil goat Myotragus. J Vertebr Paleontol. 2001;21(3):621–624. [Google Scholar]
- 41.Köhler M, Moyà-Solà S. Reduction of brain size and sense organs in the fossil insular bovid Myotragus. Brain Behav Evol. 2004;63:125–140. doi: 10.1159/000076239. [DOI] [PubMed] [Google Scholar]
- 42.Van Dam JA, et al. Long-period astronomical forcing of mammal turnover. Nature. 2006;443:687–691. doi: 10.1038/nature05163. [DOI] [PubMed] [Google Scholar]