Abstract
We report a case of a perinatally HIV-infected patient aged 9 years, who presented with right-sided hemiplegia. His initial CD4 T-cell was of 0.21% (4 cells/μL) and plasma HIV RNA virus of 185 976 copies/mL (log 5.27). Plasma and CSF samples were subsequently positive for JCV. Twelve days after the initiation of highly active antiretroviral therapy (HAART), the MRI showed progressive white matter lesions with asymmetrical deep and subcortical white matter lesions over the left frontotemporoparietal region and the right frontal lobe. Immune Reconstitution Inflammatory Syndrome (IRIS) was suspected, and the patient was treated with methylprednisolone. His clinical symptoms worsened and despite therapy the patient deteriorated.
1. Background
Progressive multifocal leukoencephalopathy (PML) is caused by the JC virus (JCV). As a demyelinating disease, PML typically presents with altered mental status, motor deficits, and ataxia, and is associated with immunosuppression, especially human immunodeficiency virus (HIV) [1]. Prevalence of JCV specific antibodies increases rapidly during childhood, but the mode of transmission is unknown [2]. PML is rare in HIV-infected children and even more uncommonly associated to immune reconstitution inflammatory syndrome (IRIS) in children.
2. Case Report
2.1. First Admission
A previously healthy 9-year-old boy (whose mother was recently found to be HIV-positive) presented to the hospital with 1 week of right-sided hemiplegia and right-sided facial palsy. Past medical history included psoriasis, diagnosed 4 years prior. His only HIV exposure was perinatal. On exam the patient weighed 22 kilograms, and vitals signs were within normal limits. He was alert and oriented with a normal level of consciousness and responded appropriately to questions. His speech was slowed and slurred, but this was his baseline according to his mother. He had right-sided facial palsy and right-sided tongue deviation; otherwise cranial nerves were intact. The boy had 3/5 strength in the right upper extremity and 4/5 strength in the right lower extremity. Left-sided strength was 5/5. The patient was able to walk with limited difficulty. Deep tendon reflexes were 2+ and 3+ throughout. Babinski's sign showed dorsiflexion of the right 1st toe. Sensation was intact throughout. The patient also had clusters of 1-2 mm skin colored papules on his forehead and left cheek in addition to diffuse mild psoriatic scaling. Immunizations were up to date. The patient was admitted for a workup of these symptoms.
Laboratory studies showed complete blood count: hematocrit 31.6%, hemoglobin 10.5 g/dL, white blood cell count 5000 cells/μL (N 41%, L 35%, E 12%, M 6%, B 1%, atypical L 1%), and 157 000 platelets/μL. HIV-antibody test was positive with a CD4 T-cell 0.21% (4 cells/μL) and plasma HIV RNA virus of 185 976 copies/mL (log 5.27). Tests for Cryptococcus antigen, Toxoplasmosis antigen, Ebstein Barr virus IgG and IgM, Cytomegalovirus IgG and IgM, Hepes Simplex virus polymerase chain reaction (PCR), Japanese encephalitis as well as cultures for tuberculosis and fungi (plasma and cerebrospinal fluid, CSF) were all negative. Plasma and CSF samples were positive for JCV by real time PCR with a plasma RNA level of 226 copies/mL.
Three days after admission a brain computerized tomography (CT) scan was performed and showed frond-like hypodense lesion at the left frontal lobe with mild effacement of the left frontal horn of the lateral ventricle. Highly active antiretroviral therapy (HAART) regimen was subsequently started 10 days after admission, consisting of GPOvir-Z (coformulated zidovudine 250 mg, lamivudine 150 mg, and nevirapine 200 mg) [3]. Clinically, the patient deteriorated during the 1st and 2nd weeks of HAART with fever and increased right leg weakness, but immunologic and virologic improvement was seen (CD4 T-cell count 0.8%, 10 cells/μL, and a plasma HIV RNA viral level of 26 532 copies/mL, log 4.42). CT (Figure 1) and brain magnetic resonance imaging (MRI; Figure 2) scans were subsequently performed. The scans showed progressive of white matter lesions with asymmetrical deep and subcortical white matter lesions over the left frontotemporoparietal region and the right frontal lobe. The lesion on the left hemisphere involved internal capsule, lentiform nucleus, thalamus, and genu of corpus callosum and anterior cerebellar hemisphere. There were no enhanced areas after the contrast study.
Figure 1.
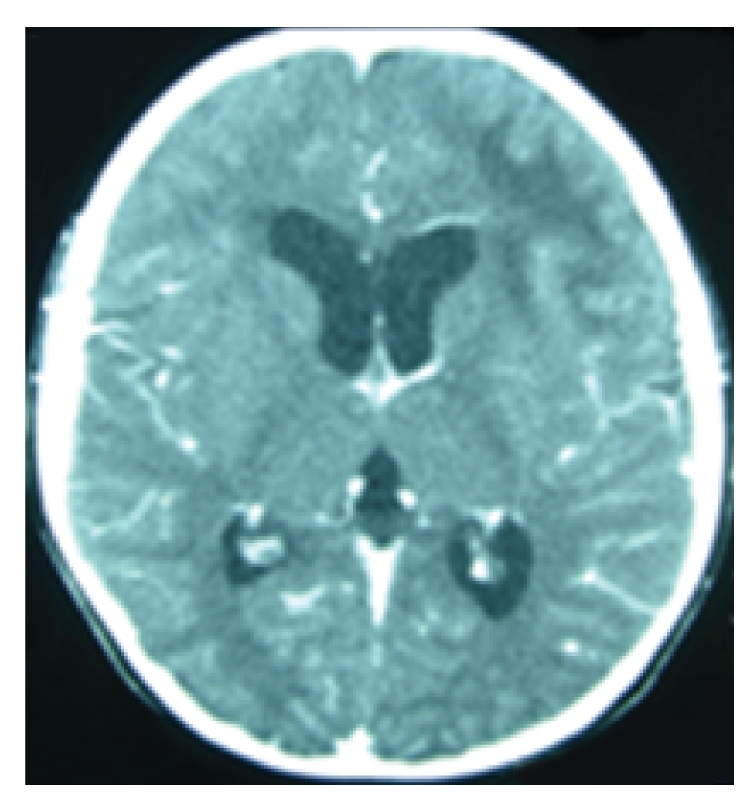
CT on day 30 of onset of symptoms and 1 week post-HAART initiation.
Figure 2.
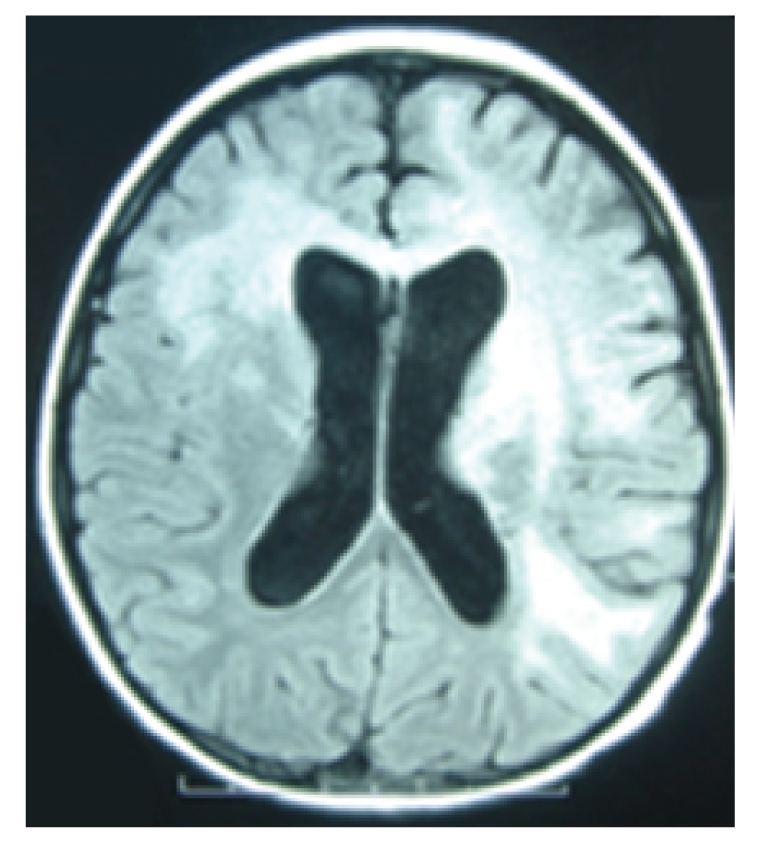
MRI on day 38 of onset of symptoms and 2 weeks post-HAART initiation.
The patient was discharged 7 weeks after the first admission. Upon discharge he was able to walk with assistance, but was unable to speak.
2.2. Second Admission
The patient was readmitted one day after discharge due to autonomic nervous system dysfunction (nausea, vomiting, and loss of bowel and bladder tone). Deep tendon reflexes were 4+ throughout, and Babinski was positive bilaterally. Continued improvement of immunologic (CD4 T-cell count 2.0%, 30 cells/μL) and virologic (HIV RNA level 3220 copies/mL, log 3.51) measures were seen. Due to progressive neurologic symptoms HAART was ceased. The second MRI (Figure 3) scan was performed and showed a progressive lesion in the same regions as described in the previous MRI, but also found new lesions over the midbrain, pons, and medulla predominantly on the left. The patient was discharged approximately 3 weeks after admission.
Figure 3.
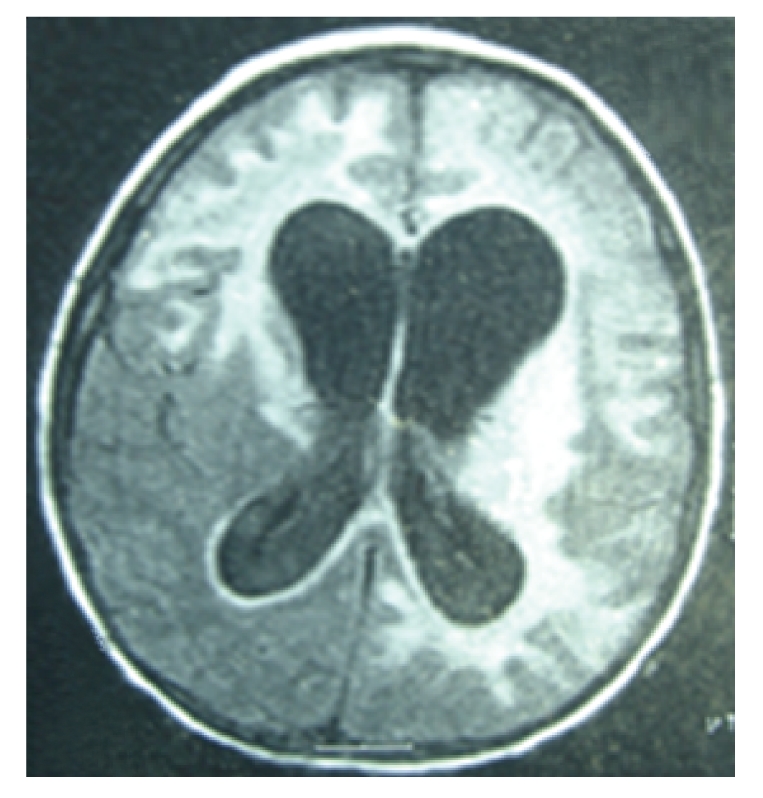
2nd MRI, 3 1/2 months after onset of symptoms, 3 days post-HAART cessation.
2.3. Third Admission
The patient was readmitted 1 month later (100 days after onset of symptoms). Immune Reconstitution Inflammatory Syndrome (IRIS) was suspected, and the boy was treated with methylprednisolone (2 mg/kg/day) for 5 days. Despite HAART suspension and administration of steroids, his clinical symptoms worsened. The third MRI (Figure 4) scan showed new lesions in the regions of the right brainstem and right hemisphere with gyral enhancement. The patient's mother refused further treatment, he was discharged home, and he subsequently died 1 year later.
Figure 4.
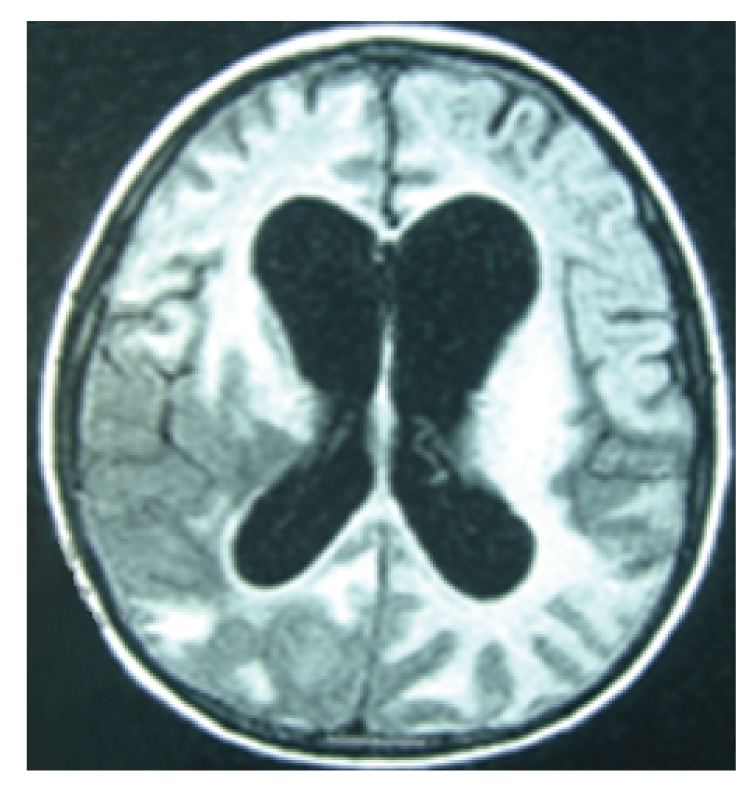
3rd MRI, 4 months after onset of symptoms, 19 days post-HAART cessation.
3. Summary
We report the 2nd case of IRIS associated PML in a perinatally HIV-infected child. Since 1992 there have been reports of 14 HIV-infected children having PML (Table 1) [4–14]. Overall, PML in HIV-infected children has occurred mostly in boys (9/14, 75%), with a median age of 10 years (range: 7–17). Reports have come from Brazil, Hungary, India, Japan, South Africa, Thailand, and USA. Presenting symptoms included: altered speech, hemiplegia, facial palsy, and cerebellar dysfunction. All had significant changes on MRI or CT. Presenting CD4 T-cell counts were low, while viral loads were high. The most common outcome was death.
Table 1.
Overview of studies concerning progressive multifocal leukoencephalopathy in HIV-infected children.
| Author | Year reported | Sex | Age (years) | Country | Presentation | MRI/CT | Previous HIV- related event | HAART regimen | CD4 T-cell % or count (cell/μL) | Viral load (copies/mL) | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Nearest to episode | Baseline | Nearest to episode | ||||||||||
| Oberdorfer et al. | 2009 | M | 9 | Thailand | Right hemiplegia | Frond-like hypodense lesion at the left frontal lobe | None | Initially nothing then AZT, 3TC, NVP | 0.21 | 0.21 | 185 976 | 185 976 | Dead |
| Liptai et al. | 2007 | M | 15 1/2 | Hungary | Dizzy, diplopia, clumsy right hand, unsteady gait | Large, nonenhancing lesion of the right cerebellar heimsphere with a slight mass effect | None reported | Initially AZT, ddl then refused HAART | 37 | 10.5 | 2900 | 256 000 | Dead |
| Shah and Chudgar. | 2005 | F | 8 1/2 | India | Right-sided dystonia, inability to talk, eat, or sit | Asymmetrical subcortical, right frontoparietal, left occipitoparietal and left vasal ganglia lesions | Generalized tonic clonic seizures and loss of consciousness | Initially nothing then AZT, 3TC, EFV, NLF | 320 | Alive, still has dystonia | |||
| Robinson et al. | 2004 | M | 17 | USA | Dysarthric speech, facial palsy | Multiple confluent areas of high signal and fluid attenuated inversion recovery in the corona radiata bilaterally | Pneumocystis jiroveci pneumonia | Initially AZT, 3TC then d4T, NVP, lopinavir/ ritonavir then included cidofovir | 3 | 3 | 10–90 000 | 29 100 | Stable, wide-based gait, dysarthria, right-sided tongue deviation |
| Nuttall et al. | 2004 | M | 12 | South Africa | Acute cerebellar dysfunction, hemiparesis | Nonenhancing low density lesion in the left cerebellar hemisphere | Growth failure, chronic lung disease | d4T, 3TC, efavirenz | 1.08 | 5.45 | 96 000 | Undetectable | Stable, mild cerebellar dysfunction |
| Inui et al. | 1999 | M | 12 | Jaban | Left upper extremity weakness | White matter lesions of the right frontal, parietal and occipital lobes | Candida stomatitis | AZT then ritonavir and 3TC added | 9.5 | 7600 | Stable, left hemiparesis | ||
| Araujo et al. | 1997 | M | 10 | Brazil | Subacute cerebellar dysfunction, dementia | Focal nonenhancing area of low attenuation in the cerebellum | None reported | ||||||
| Morriss et al. | 1997 | M | 7 | USA | Decreased activity, slurred speech, ataxia | Confluent, nonenhancing, low density lesion in the right cerebellar white matter, middle cerebellar peduncle and dorsolateral pons | Candida esophagitis | ddc | 0 | Death | |||
| Whiteman et al. | 1993 | M | 10 | USA | |||||||||
| F | 12 | USA | Increased signal intensity in the basal ganglia and corona radiata bilaterally | ||||||||||
| Berger et al. | 1992 | F | 13 | USA | Dysarthria, paresthesias of tongue and chin | Sinusitis and hyperintense signals in the basal ganglia | Oral candidiasis | AZT | 421 | 7 | Death | ||
| M | 10 | USA | Facial palsy | Left frontal lobe lesion | Pneumocystis jiroveci pneumonia | AZT | 100 | Death | |||||
| Vandersteenhoven et al. | 1992 | M | 7 | USA | Decreased activity, left hemiparesis, falling | Bilateral confluent abnormal white matter hyperintensity in the region of the right subcortical/ periventricular region | None reported | Initially nothing then AZT | 390 | Death | |||
Neuroimaging is an important part of the diagnosis. Multiple bilateral areas of white matter demyelination without contrast enhancement or mass effect are typical findings. For CT imaging these appear hypodense, while on MRI they have either decreased or increased signals depending on the imaging parameters [14–16]. Treatment for PML is based on HAART initiation or optimization, which has shown improved mortality associated with lower HIV RNA plasma viral levels and higher CD4 T-cell count [17–20].
Although rare, casesof PML associated with IRIS occur where there is clinical deteriorationdespite improvement of immunological and virologicalmeasures after the initiation of HAART [21, 22]. This was seen in the case reported here and the other reported case of PML in an HIV-infected child associated with IRIS [10]. Reported in 2004, a 12-year-old African boy developed cerebellar dysfunction and hemiparesis 5 weeks after starting HAART. He was started on prednisone and continued on HAART. He subsequently had immunologic and virologic improvement with full clinical recovery. Estimates from HIV-infected adults with PML associated IRIS range from 9–19%, typically occurring 3–5 weeks after initiation; this is purportedly much less common in children, as it is only sporadically mentioned in the literature [18, 23]. Therapy for PML associated IRIS has included glucocorticoids in addition to HAART interruption, which have been shown to be both beneficial and to be of no benefit in HIV-infected adults with PML [10, 22, 24–26]. Our patient saw further clinical deterioration, despite a trial of these measures unlike the boy in Africa [10].
PML in HIV-infected adolescents has a wide distribution of ages and geography. Despite the cases presented here, there is limited information about this disease in children. Underdiagnosis is likely to both perpetuate this knowledge gap and discourage physicians from identifying this condition. Additionally, in developing countries, such as Thailand, lack of imaging and laboratory data may further hinder diagnosis. Clinicians should then be cognizant of both of this condition and sequelae after HAART, so that prompt diagnosis and treatment can be made. Clear guidelines would be beneficial to clinicians who face these complex patients.
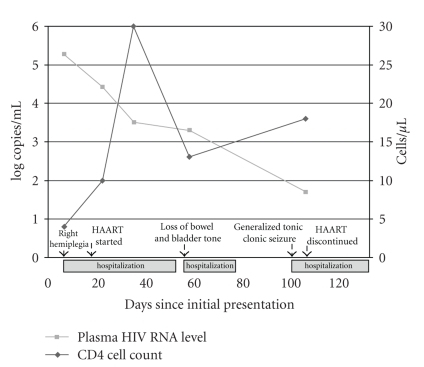
Figure 5.
Clinical manifestations and plasma HIV RNA and CD4 cell count levels.
Acknowledgments
The authors would like to thank Dr. Virat Sirisanthana who provided valuable advice. This work was supported by Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (to P. Oberdorfer, K. Katanyuwong, and P. Jpttamala) and by a grant from the NIH/Fogarty Clinical Research Training Scholars Program (to C.H. Washington).
Abbreviations
- 3TC:
Lamivudine;
- AZT:
Ziduvodine;
- d4T:
Stavudine;
- ddC:
Zalcitabine;
- ddI:
Didanosine;
- EFV:
Efavirenz;
- HAART:
Highly active antiretroviral therapy;
- NLF:
Nelfinavir;
- NVP:
Nevirapine.
References
- 1.Weber T. Progressive multifocal leukoencephalopathy. Neurologic Clinics. 2008;26(3):833–854. doi: 10.1016/j.ncl.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Padgett BL, Walker DL. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. Journal of Infectious Diseases. 1973;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- 3.GPO . GPOvir-Z. Bangkok, Thailand: Government Pharmaceutical Company; [Google Scholar]
- 4.Araujo AP, Pereira HS, Oliveira RH, Frota AC, Esperanca JC, Duarte F. Progressive multifocal leukoencephalopathy in a child with acquired immunodeficiency syndrome (AIDS) Arquivos de Neuro-Psiquiatria. 1997;55(1):122–125. doi: 10.1590/s0004-282x1997000100019. [DOI] [PubMed] [Google Scholar]
- 5.Berger JR, Scott G, Albrecht J, Belman AL, Tornatore C, Major EO. Progressive multifocal leukoencephalopathy in HIV-1-infected children. AIDS. 1992;6(8):837–841. doi: 10.1097/00002030-199208000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Inui K, Miyagawa H, Sashihara J, et al. Remission of progressive multifocal leukoencephalopathy following highly active antiretroviral therapy in a patient with HIV infection. Brain and Development. 1999;21(6):416–419. doi: 10.1016/s0387-7604(99)00038-8. [DOI] [PubMed] [Google Scholar]
- 7.Krasinski K, Borkowsky W, Holzman RS. Prognosis of human immunodeficiency virus infection in children and adolescents. Pediatric Infectious Disease Journal. 1989;8(4):216–220. [PubMed] [Google Scholar]
- 8.Liptai Z, Papp E, Barsi P, et al. Progressive multifocal leukoencephalopathy in an HIV-infected child. Neuropediatrics. 2007;38(1):32–35. doi: 10.1055/s-2007-981482. [DOI] [PubMed] [Google Scholar]
- 9.Morriss MC, Rutstein RM, Rudy B, Desrochers C, Hunter JV, Zimmerman RA. Progressive multifocal leukoencephalopathy in an HIV-infected child. Neuroradiology. 1997;39(2):142–144. doi: 10.1007/s002340050383. [DOI] [PubMed] [Google Scholar]
- 10.Nuttall JJC, Wilmshurst JM, Ndondo AP, et al. Progressive multifocal leukoencephalopathy after initiation of highly active antiretroviral therapy in a child with advanced human immunodeficiency virus infection: a case of immune reconstitution inflammatory syndrome. Pediatric Infectious Disease Journal. 2004;23(7):683–685. doi: 10.1097/01.inf.0000130954.41818.07. [DOI] [PubMed] [Google Scholar]
- 11.Robinson L-G, Chiriboga CA, Champion SE, Ainyette I, Abrams EJ. Progressive multifocal leukoencephalopathy successfully treated with highly active antiretroviral therapy and cidofovir in an adolescent infected with perinatal human immunodeficiency virus (HIV) Journal of Child Neurology. 2004;19(1):35–38. doi: 10.1177/088307380401900107011. [DOI] [PubMed] [Google Scholar]
- 12.Shah I, Chudgar P. Progressive multifocal leukoencephalopathy (PML) presenting as intractable dystonia in an HIV-infected child. Journal of Tropical Pediatrics. 2005;51(6):380–382. doi: 10.1093/tropej/fmi034. [DOI] [PubMed] [Google Scholar]
- 13.Vandersteenhoven JJ, Dbaibo G, Boyko OB, et al. Progressive multifocal leukoencephalopathy in pediatric acquired immunodeficiency syndrome. The Pediatric Infectious Disease Journal. 1992;11(3):232–237. doi: 10.1097/00006454-199203000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Whiteman MLH, Post MJD, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology. 1993;187(1):233–240. doi: 10.1148/radiology.187.1.8451420. [DOI] [PubMed] [Google Scholar]
- 15.Donovan Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? American Journal of Neuroradiology. 1999;20(10):1896–1906. [PMC free article] [PubMed] [Google Scholar]
- 16.Skiest DJ. Focal neurological disease in patients with acquired immunodeficiency syndrome. Clinical Infectious Diseases. 2002;34(1):103–115. doi: 10.1086/324350. [DOI] [PubMed] [Google Scholar]
- 17.Antinori A, Cingolani A, Lorenzini P, et al. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA) Journal of NeuroVirology. 2003;9(supplement 1):47–53. doi: 10.1080/13550280390195388. [DOI] [PubMed] [Google Scholar]
- 18.Berenguer J, Miralles P, Arrizabalaga J, et al. Clinical course and prognostic factors of progressive multifocal leukoencephalopathy in patients treated with highly active antiretroviral therapy. Clinical Infectious Diseases. 2003;36(8):1047–1052. doi: 10.1086/374048. [DOI] [PubMed] [Google Scholar]
- 19.De Luca A, Giancola ML, Ammassari A, et al. The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS-associated progressive multifocal leukoencephalopathy. Journal of Infectious Diseases. 2000;182(4):1077–1083. doi: 10.1086/315817. [DOI] [PubMed] [Google Scholar]
- 20.Marra CM, Rajicic N, Barker DE, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS. 2002;16(13):1791–1797. doi: 10.1097/00002030-200209060-00012. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann C, Horst H-A, Albrecht H, Schlote W. Progressive multifocal leucoencephalopathy with unusual inflammatory response during antiretroviral treatment. Journal of Neurology Neurosurgery and Psychiatry. 2003;74(8):1142–1144. doi: 10.1136/jnnp.74.8.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Safdar A, Rubocki RJ, Horvath JA, Narayan KK, Waldron RL. Fatal immune restoration disease in human immunodeficiency virus type 1-infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy-associated immune reconstitution. Clinical Infectious Diseases. 2002;35(10):1250–1257. doi: 10.1086/344056. [DOI] [PubMed] [Google Scholar]
- 23.Cinque P, Bossolasco S, Brambilla AM, et al. The effect of highly active antiretroviral therapy-induced immune reconstitution on development and outcome of progressive multifocal leukoencephalopathy: study of 43 cases with review of the literature. Journal of NeuroVirology. 2003;9(supplement 1):73–80. doi: 10.1080/13550280390195351. [DOI] [PubMed] [Google Scholar]
- 24.Martinez JV, Mazziotti JV, Efron ED, et al. Immune reconstitution inflammatory syndrome associated with PML in AIDS: a treatable disorder. Neurology. 2006;67(9):1692–1694. doi: 10.1212/01.wnl.0000242728.26433.12. [DOI] [PubMed] [Google Scholar]
- 25.Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F. Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathologica. 2005;109(4):449–455. doi: 10.1007/s00401-005-0983-y. [DOI] [PubMed] [Google Scholar]
- 26.Venkataramana A, Pardo CA, McArthur JC, et al. Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology. 2006;67(3):383–388. doi: 10.1212/01.wnl.0000227922.22293.93. [DOI] [PubMed] [Google Scholar]