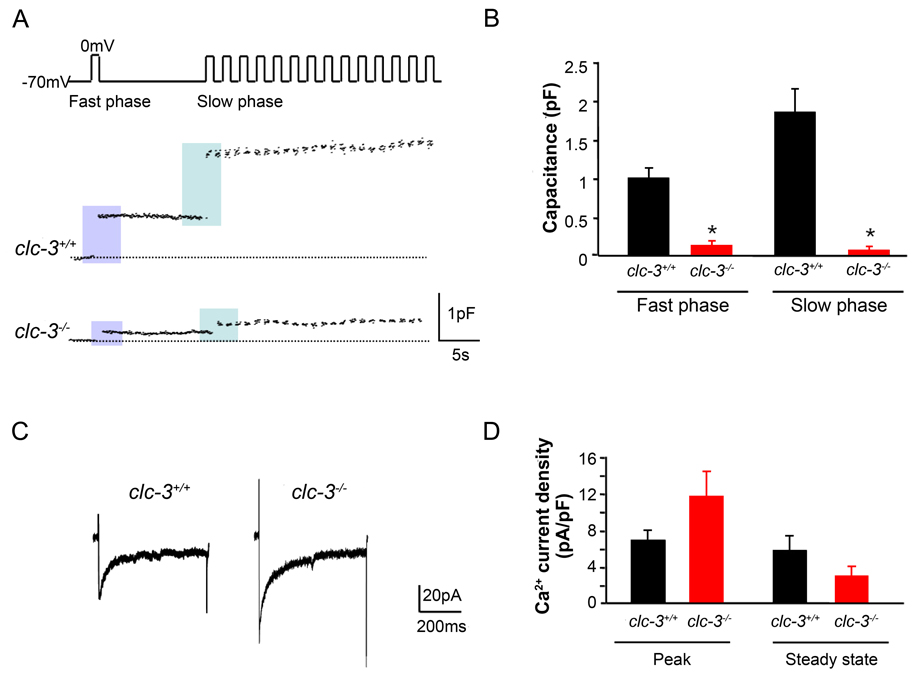
Figure 2. Reduction in depolarization-induced exocytosis in pancreatic β–cells isolated from clc-3−/− mice compared to clc-3+/+.
(A) Perforated patch recordings from representative pancreatic β–cells showing increases in cell membrane capacitance (ΔCm) evoked by voltage-clamp depolarizations from −70 to 0 mV (500 ms, 1 Hz). (B) Histograms showing average changes in capacitance during fast and slow phase of secretion. Fast phase secretion (from a docked, readily releasable granule pool) was triggered by a single pulse from −70 to 0 mV (500 ms) 10 s before the train was applied to the cells to elicit mobilization of granules from a reserve pool of granules (slow phase). Data are mean ± S.E.M. of 4 experiments for each cell type. * P < 0.05. (C) Representative whole-cell Ca2+ currents from perforated patch capacitance experiments as in (A). Currents were elicited by 500 ms voltage-clamp depolarizations from −70 to 0 mV. (D) Summary of average changes in peak and steady-state Ca2+ current in clc-3+/+ and clc-3−/− cells. Neither average peak nor steady-state currents were significantly different from one another. Data are mean ± S.E.M. of 4 experiments for each cell type.