Fig.3.
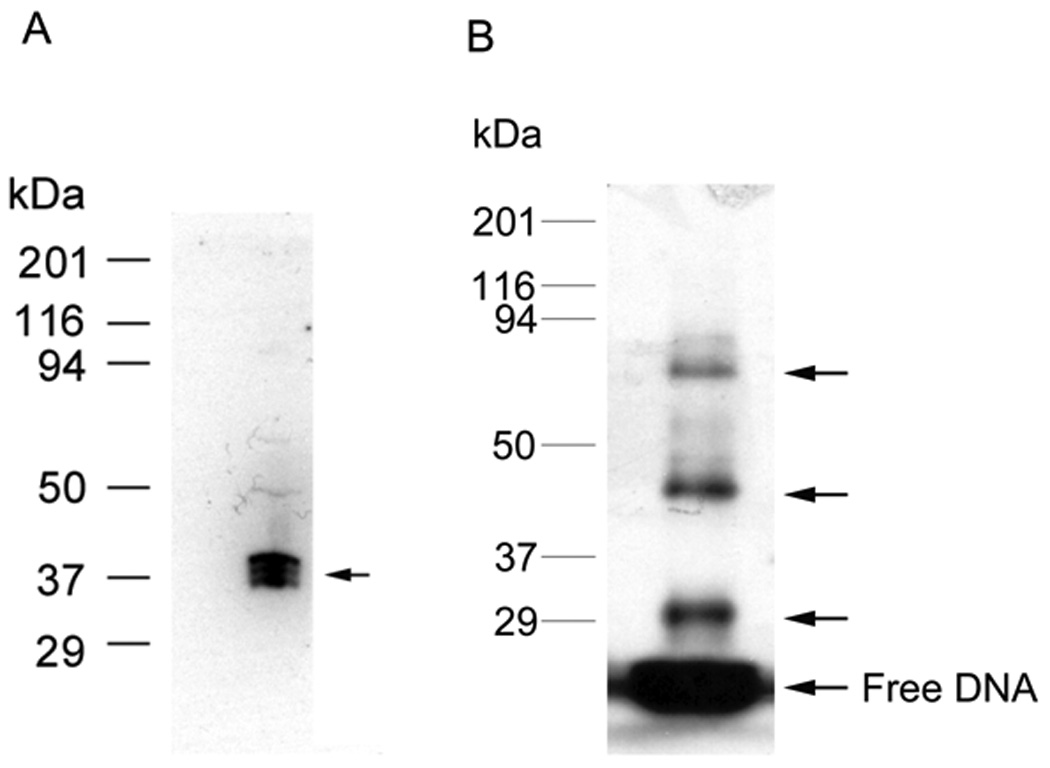
(A) Southwestern blot assay. HEK293 nuclear extract was separated by 12% SDS-PAGE gel electrophoresis and transferred to nitrocellulose membrane. The blotted proteins were denatured and renatured by adding guanidine hydrochloride to 6M and gradual dilution to 0.1M. The proteins on blot bound the labeled c-jun element (1.5 nM) during subsequent incubation and washing. Autoradiography analysis indicates the position of proteins on the blot that interacted with element DNA, indicated by the arrow. In this case, the complex consists of three closely spaced bands from 37–40 kDa. (B) DNA UV-crosslinking assay in vitro. HEK293 nuclear extract was incubated with labeled WT c-jun element at room temperature for 30 min, then UV crosslinked on ice for another 30 min, the UV-crosslinked proteins are separated by 12% SDS-PAGE gel electrophoresis. Autoradiography analysis indicates the position of protein-DNA complex, indicated by arrows. This revealed a protein DNA complex of about 45 and 85 kDa in this case plus a DNA-DNA crosslink near 30 kDa. Protein standards are shown on the left.
