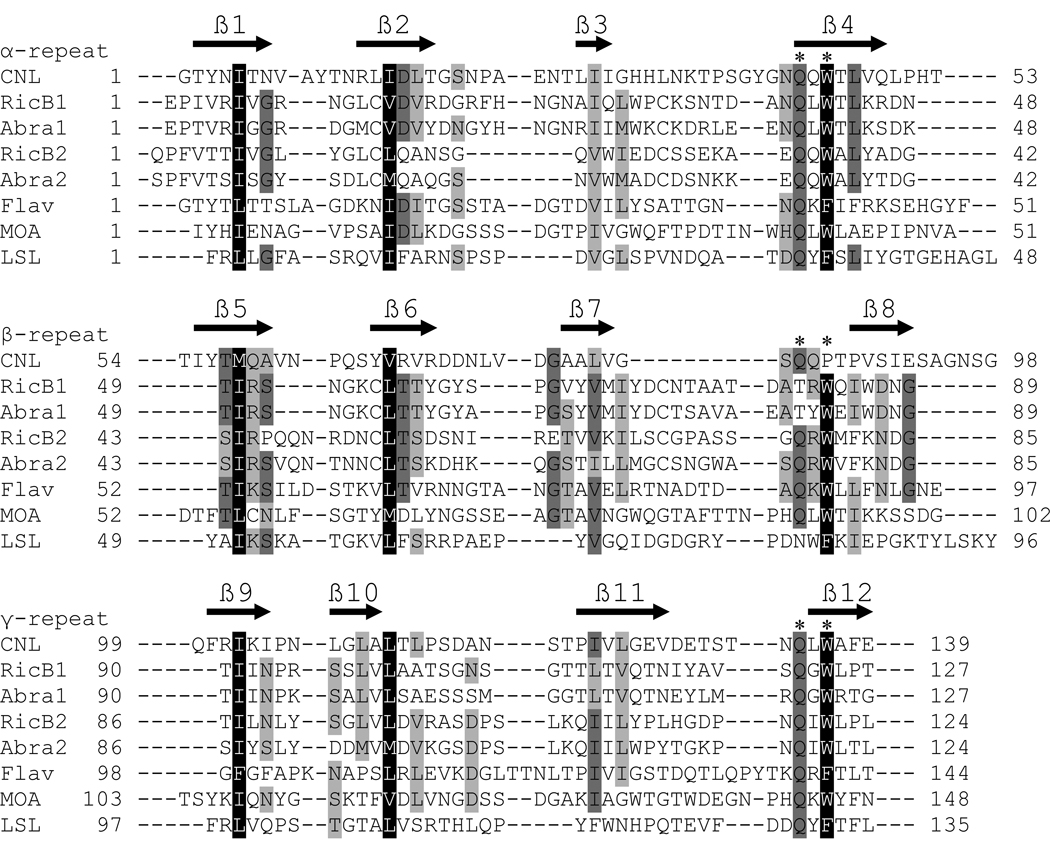
Fig. 2.
Sequence alignment of the CNL ricin B-like lectin domain with representative ricin B-type lectin domains of proteins with known structures. RicB1, ricin B chain lectin domain 1, and RicB2, ricin B chain lectin domain 2 (Swiss-Prot P02879); Abra1, abrin-a B chain lectin domain 1, and Abra2, abrin-a B chain lectin domain 2 (P11140); Flav, flavastacin (Q47899); MOA, Marasmius oreades agglutinin (Q8X123); LSL, Laetiporus sulphureus lectin (Q7Z8V1). Identical amino acid residues are shaded dark gray, conserved hydrophobic residues forming a hydrophobic core are shaded black and other similar residues are shaded light gray. The ricin B-type domains consist of three homologous repeats (α, β, and γ; denoted above sequence names), each containing the QxW motif indicated by two asterisks above the sequence (* *). The predicted β-strands of CNL secondary structure are indicated by black arrows above the amino acid sequence.